��Ŀ����
����Ŀ��������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ)��

ʵ��������£�
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ�У�
�ڿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ���(65%HNO3��98%H2SO4��������Ϊ2��1.5)��Һ��
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�壻
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6+12HNO3��3H2C2O4+9NO2��+3NO��+9H2O
C6H12O6+8HNO3��6CO2+8NO��+10H2O
3H2C2O4+2HNO3��6CO2+2NO��+4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����_________��
(3)װ��C����β�����գ���β����n(NO2)��n(NO)=1��1ʱ��������NaOH��Һ�ܽ�NO��ȫ�����գ�ԭ����_________(�û�ѧ����ʽ��ʾ)��
(4)����NaOH��Һ����β����Ƚϣ����õ���ˮ��Һ����β�������š�ȱ����________��
(5)�����ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ�������_________��
���𰸡���ˮ �����¶ȹ��ߡ�����Ũ�ȹ�����C6H12O6��H2C2O4��һ�������� NO2+NO+2NaOH=2NaNO2+H2O �ŵ㣺���HNO3�����ʣ�ȱ�㣺NOx���ղ���ȫ ����©��������ƿ
��������
(1)���ݵ��۵����Է������
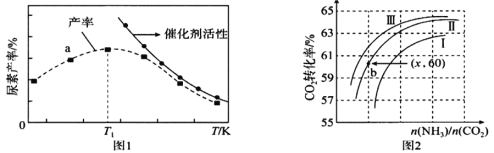
(2)����������Ǿ����л�ԭ�ԣ������¶ȣ���������Ũ�ȣ������������Խǿ��
(3)NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£�������̬���з�Ӧ��
(4)����β���ijɷֺ��������ƺ͵��۵����ʽ��
(5)���ݼ�ѹ���˵��ص�������
(1)�����������ɫ�����Ѿ�ˮ��ĵ�����Һ�еμӼ��ε�Һ����Һ����ɫ����֤������û����ȫˮ�⣻��Һ������ɫ����֤��������ȫˮ�⣬�ʴ�Ϊ����ˮ��
(2)����Ϊ65%HNO3��98%H2SO4�Ļ��Һ�����Һ����ˮ���ȣ��¶ȸ��ܼӿ컯ѧ��Ӧ��������μӹ��죬����Ũ�ȹ�����C6H12O6��H2C2O4��һ�����������ʴ�Ϊ���¶ȹ��ߡ�����Ũ�ȹ�����C6H12O6��H2C2O4��һ����������
(3) NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£����߷�����Ӧ����NaNO2����Ӧ�Ļ�ѧ����ʽΪNO+NO2+2NaOH=2NaNO2+H2O���ʴ�Ϊ��NO2+NO+2NaOH=2NaNO2+H2O��
(4)β��Ϊһ�������Ͷ����������ü����գ����ǽ�ת��Ϊ����������ȫ���գ�����õ���ˮ��Һ���գ�����������ˮ��Ӧ�������ᣬ�������ܼ�����������������NOx���ղ���ȫ���ʴ�Ϊ���ŵ㣺���HNO3�����ʣ�ȱ�㣺NOx���ղ���ȫ��
(5)��ѹ�����볣ѹ������ȣ��ŵ㣺�ɼӿ�����ٶȣ����ܵõ��ϸ���ij�����װ���ص㣺����©������б��ҪԶ������������ƿ�ij����죬��˲����ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ������в���©��������ƿ���ʴ�Ϊ������©��������ƿ��

����Ŀ���������������������������������ӦΪ��2NO2(g)+O3(g)![]() N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����( )
N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����( )
A | B | C | D |
|
|
|
|
�����¶ȣ�����Ӧ�����Ƿ��ȷ�Ӧ | 0~3s�ڣ���Ӧ����Ϊ��v(NO2)��0.2 mol��L-1��s-1 | t1ʱ�����������ƽ�������ƶ� | ��ƽ��ʱ�����ı�x����xΪc(O2) |
A.AB.BC.CD.D
����Ŀ��H2O2����ȡ��������ˮ���������Ӧ���ǵ�ǰ��ѧ�о����ȵ㡣 �ش��������⣺

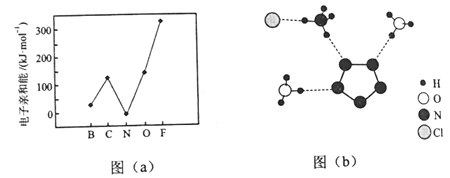
��1��������ͬ��������������������[��NH4��2S2O8]��ԭ����ͼ��ʾ����������������Ӧ��������_______�������ĵ缫��ӦʽΪ_________��
��2��100��ʱ���ڲ�ͬ�������Ӵ����£�����������24h�ķֽ��ʼ��±���
���� | ������/��mg��L-1�� | �ֽ���/% | ���� | ������/��mg��L-1�� | �ֽ���/% | |
�� | �� | 2 | Fe3+ | 1.0 | 15 | |
Al3+ | 10 | 2 | Cu2+ | 0.1 | 86 | |
Zn2+ | 10 | 10 | Cr3+ | 0.1 | 96 |
���ϱ����ݿ�֪����ʹ��������ֽⷴӦ��ܽ�������������_______�����˹�������ʱ����ѡ�õ���������Ϊ________�����ţ���
A ���� B ��ͭ C ���� D �����
��3���������������£�H2O2��һ�ִ��ֽ�������£�
H2O2��aq����Mn2+��aq��=OH��aq����Mn3+��aq����OH����aq�� ��H��a kJ/mol
H2O2��aq����Mn3+��aq����2OH����aq��=Mn2+��aq������O2- ��aq����2H2O��l�� ����b kJ/mol
OH��aq������O2-��aq��=O2��g����OH����aq�� ��H��c kJ/mol
��2H2O2��aq��=2H2O��l����O2��g������H��_________���÷�Ӧ�Ĵ���Ϊ________��
��4��298 Kʱ����10 mL a mol��L1 NaH2PO2��10 mL 2a mol��L1 H2O2��Һ��10 mL NaOH��Һ��ϣ�������Ӧ��H2PO2-��aq����2H2O2��aq����2OH��aq��![]() PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
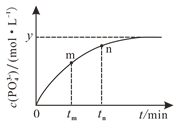
�����п��жϷ�Ӧ�ﵽƽ�����_______�����ţ���
a c��H2PO2-����y mol��L1
b ��Һ��pH���ٱ仯
c v��H2O2����2v��H2PO2-��
d c��PO43-��/c��H2PO2-�����ٱ仯
��tmʱv��_____tnʱv����������������С������������������
����ƽ��ʱ��Һ��pH��12����÷�Ӧ��ƽ�ⳣ��KΪ___________��