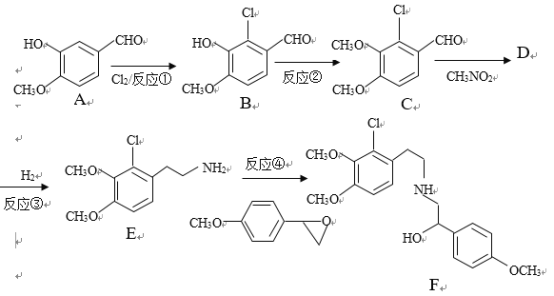
��Ŀ����
����Ŀ����һNa2SO3�����ֱ����������ʡ�Ϊ�ⶨ��Ʒ���ȣ��ס�����λͬѧ�ֱ����������ʵ�鷽����
����ͬѧ����ȡm g��Ʒ����ˮ�����������BaCl2��Һ�����ˣ�������м���������ᣬ�ٹ��ˣ�����������ϴ�Ӻ���к�ɣ���ȴ��Ƶ�����Ϊm1 g��
(1)BaCl2��Һ���������ԭ����___________________________________________������Һ�еμ�____________��Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣬�����������Һ�м���BaCl2��Һ����й��ˡ�ϴ�Ӳ�������ķ�����__________________________________________________����ϴ��Һ�еμ�_______________��Һ�������жϲ��������Ƿ�ϴ�Ӹɾ���
(2)�жϲ�����������m1 g�ܹ���Ϊʵ��ⶨֵ�������ʽ��������Ʒ���ȣ����벹���ʵ�������_________________________________________________________��
����ͬѧ����ȡm g��Ʒ����ˮ�������Һ���õζ���ȡV mL����ƿ�У���Ũ��Ϊc mol/L�ı����Ը��������Һ�ζ����յ㡣��Ӧ��ϵΪ��SO32�� + MnO4�� SO42�� + Mn2+ (δ��ƽ)
(3)������Ʒ��Һʱ����һ����Ҫ��ʵ��������__________(ѡ��𰸱��)��
a������ƿ b�������� c���ζ��� d���ձ�
(4)�ζ��յ���ж�������___________________________________________________��
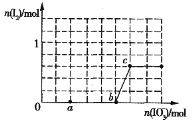
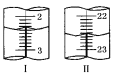
(5)�����ζ��У��ζ�����ע�����Ը��������Һ֮ǰ����������ˮϴ��������__________________________________________���ζ���Һ��仯��ͼ��ʾ����ͼ��ͼ����ʾ���ĵĸ��������Һ���Ϊ__________________��
���𰸡�ȷ��SO42-������ȫ Na2CO3 �ڹ������м�ˮ����û���壬ʹ����Ȼ���£��ظ�2~3�� AgNO3 �ظ���ɡ���ȴ������������������m1�IJ�ֵС��0.001 g c �������һ�����Ը��������Һ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ���Ӳ���ɫ �����������Һ��ϴ2~3�� 20.20 mL
��������
ʵ���м��������BaCl2��Һ��Ŀ����ʹ����������ܳ�ֳ���������Һ�еμ�̼������Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣻��ϴ�ӵõ������ᱵ������ϴ�ӹ���ķ���ͬ���ˣ��ڹ������м�������ˮ����û�����壬Ȼ����ˣ�ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ��������ʵ����������ӵĴ����������ȷ�����ɵ����ᱵ������ȫ��ɣ�������������m1�IJ�ֵС��0.001g������һ�����ʵ���Ũ�ȵ���Һʱ��Ҫ�õ�����ƿ�����������ձ��������õζ��ܣ��ݴ˴��⣻�ø��������Һ�ζ���������Һʱ���ڵζ��յ㣬��Һ����ɫ����ɫ�����ݵζ��ܵ�ʹ�÷�����֪���ζ�����װ��Һǰ�����ô�װҺ��ϴ�����ݵζ��ܹ��켰ͼʾ�ζ�����Һ��������ĵĸ��������Һ�����
(1)ʵ���м��������BaCl2��Һ��Ŀ����ʹ����������ܳ�ֳ���������Һ�еμ�̼������Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣻ϴ�ӹ���ķ���ͬ���ˣ��ڹ������м�������ˮ������û���壬ʹ����Ȼ���£��ظ�23�Σ�ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ�������������Ϊ�������ϴ�ӹ��˳�����Һ�еμ���������Һ���������ɣ�����������ϴ����
�ʴ�Ϊ��ʹ����������ܳ�ֳ�����̼���ƣ��ڹ������м�������ˮ������û���壬ʹ����Ȼ���£��ظ�23�Σ���������
(2) �жϲ�����������m1g�ܹ���Ϊʵ��ⶨֵ�������ʽ��������Ʒ����,�����ظ���ɡ���ȴ����������ɺ������������m1�IJ�ֵС��0.001g��
�ʴ�Ϊ���ظ���ɡ���ȴ������,����������m1�IJ�ֵС��0.001g��
(3) ��������һ�����ʵ���Ũ�ȵ���Һ�����У���Ҫʹ�õ������У�һ���ݻ�������ƿ�����������ʱ�õ����������ܽ������Ҫ���ձ��н��У�����ʱ��Ҫʹ�ý�ͷ�ιܣ�������Ҫ�������У�a.����ƿ��b.��������d.�ձ���e.��ͷ�ιܣ�һ�㲻��Ҫ��Ϊc.�ζ��ܣ�
�ʴ�Ϊ��c��
(4) �ø��������Һ�ζ���������Һʱ�����������һ�����Ը��������Һ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ����˵���Ѿ��ﵽ�ζ��յ㣬
(5) ���ݵζ��ܵ�ʹ�÷�����֪���ζ�����ע�����Ը��������Һ֮ǰ����������ˮϴ�������ñ����������Һ��ϴ23�Σ��ζ��ܵ�ÿ��С�̶�Ϊ0.10mL������ͼ2��ʾ��ͼI��ʾ������Ϊ2.40mL��ͼII��ʾ��ĩ����Ϊ22.60mL���ζ��ܹ����ĵĸ��������Һ�����Ϊ��(22.602.40)mL=20.20mL��
�ʴ�Ϊ�������������Һ��ϴ23�Σ�20.20mL��

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�