��Ŀ����
����Ŀ����������һ�ִ��Բ��ϣ����й㷺��Ӧ�á�
(1)��̬��ԭ�ӵĺ�������Ų�ʽΪ��Ar��_____________��
(2)��ҵ�Ʊ������峣ʹ��ˮ�ⷨ���Ʊ�ʱ����������[CO(NH)2]2�������Ƶȼ������ʣ����ط��������ֲ�ͬԪ�صĵ縺���ɴ�С��˳����_______����������̼ԭ�ӵ��ӻ�������_________��
(3)Fe3O4����������������ܣ��ڴ��Բ��ϵ�����Ӧ�ù㷺����ѧ�����������Ʊ�Fe3O4������õķ���֮һ�������ǽ�FeSO4��FeCl3��Һ��1��2Ͷ�ϱȻ�ϣ��ټ���NaOH��Һ�����ɲ���Fe3O4��������÷�Ӧ�����ӷ���ʽΪ______________��
(4)����Fe3O4�ľ�����ͼ��ʾ���þ�����һ�ִ��Բ��ϣ��ܵ��硣

�پ������������Ӵ���������Χ�ɵ�___________(��ռ�ṹ)��϶��
�ھ����������ӵĶѻ���ʽ��ij��������ԭ�Ӷѻ���ʽ��ͬ���öѻ���ʽ����Ϊ________��
�۽���Fe3O4�����ܵ����ԭ��_________________________������������Խ��߳�Ϊa mm����Fe3O4������ܶ�Ϊ________(�����ӵ�������NA��ʾ)g��cm��3
���𰸡�3d64s2 O>N>C>H sp3�ӻ���sp2�ӻ� Fe2++2Fe3++8OH-=Fe3O4��+4H2O �������� �����������ܶѻ� ���ӿ������ֲ�ͬ��̬�������Ӽ���ٷ���ת�� ![]()
��������
��1�����ݺ�������Ų����ɽ�������
��2���ǽ�����Խǿ���縺��Խǿ���ݴ˷��������ݴ���ṹʽ ��֪̼�������ӻ���ʽ��
��֪̼�������ӻ���ʽ��
��3���ҳ���Ӧ���������ٸ���Fe2+��Fe3+=1��2������ƽ��
��4���ٹ۲�ͼʾ��ͼ��������ʾFe2+�����ĸ������ӣ������ṹ��
�����������ѻ��ǰ˸������һ�����ģ������������ܶѻ��ǰ˸�������������ģ�ϸ�Ĺ۲죬��������߲�һ������ȥ�۲죻
�۾�������Fe2+��Fe3+��Fe2+ʧ����ת��ΪFe3+��Fe3+�õ���ת��ΪFe2+�����ݾ�̯��ȥ���㾧���ܶȡ�
��1�����ĺ����������Ϊ26�����̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]3d64s2 ���ʴ�Ϊ3d64s2��
��2������[CO(NH2)2 ]��������Ԫ�طֱ�ΪN��H��C��O��Ԫ�صķǽ�����Խǿ���縺��Խ��������Ԫ�صĵ縺���ɴ���С��˳����O>N>C>H��CH3COONa�м��е�Cԭ��Ϊ sp�ӻ����Ȼ��е�Cԭ��Ϊsp�ӻ����ʴ�Ϊ��O>N>C>H��sp3�ӻ���sp2�ӻ���
��3��FeSO4��FeCl3��Һ��1��2Ͷ�ϱȻ�ϣ��ټ���NaOH��Һ�����ɲ���Fe3O4�����������غ㷨��֪��Ӧ�ķ���ʽΪ��Fe2++2Fe3++8OH-=Fe3O4��+4H2O��
��4���ٹ۲�ͼʾ��֪��Fe2+�����ĸ������ӣ��ĸ�������Ϊ�������嶥�㣬Fe2+�������������ģ��ʴ�Ϊ���������壻
����ͼ�о������һ�������ᷢ���������ǰ˸�������������ģ����Զѻ���ʽΪ�����������ܶѻ����ʴ�Ϊ�������������ܶѻ���
�۵��ӵĵ�ʧת����ʹ�������磬������������������ܵ����ԭ���ǵ��ӿ������ֲ�ͬ��̬�������Ӽ���ٷ���ת�ƣ����ݾ����ľ�̯���㣬�����к��е������ӵĸ���Ϊ4��1/8+3��1/2=2���������ӵĸ���Ϊ1�������ӵĸ���Ϊ1+12��1/4=4����������Խ��߳�Ϊa nm����߳�Ϊx nm����Խ���Ϊ![]() ������Խ��߳�Ϊ
������Խ��߳�Ϊ![]() ����
����![]() �����Ϊ
�����Ϊ![]() ������Ϊ
������Ϊ![]() �����ܶ�
�����ܶ�![]() ��
��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�����Ŀ�������������ڲ��Ϸ����Ӧ��Խ��Խ�㷺��
(1)�װ�(CH3NH2)�Ǻϳ�̫������������ԭ�ϡ���ҵ�ϳɼװ�ԭ����
CH3OH(g)+NH3(g)![]() CH3NH2(g)+H2O(g)��H��
CH3NH2(g)+H2O(g)��H��
����֪����ָ�Ͽ�1mol��̬�������յ��������γ�1mol��̬�����ͷŵ����������ֻ�ѧ���ļ������±���ʾ��
��ѧ�� | C-H | C-O | H-O | N-H | C-N |
����/kJ��mol-1 | 413 | 351 | 463 | 393 | 293 |
��úϳɷ�Ӧ�ġ�H=______________��
��һ�������£��������ͬ�ļס��ҡ��������ĸ������У���ʼͶ���������£�
NH3(g)/mol | CH3OH(g)/mol | ��Ӧ���� | |
�� | 1 | 1 | 498K������ |
�� | 1 | 1 | 598K������ |
�� | 1 | 1 | 598K����ѹ |
�� | 2 | 3 | 598K������ |
�ﵽƽ��ʱ���ס��ҡ������������е�CH3OHת�����ɴ�С��˳��Ϊ_______________��
(2)��ҵ��������(Ga)��NH3�ڸ����ºϳɹ���뵼����ϵ�����(GaN)���䷴Ӧԭ��Ϊ2Ga(s)+2NH3(g)![]() 2GaN(s)+3H2(g)��H=-30.81kJ��mol-1��
2GaN(s)+3H2(g)��H=-30.81kJ��mol-1��
�����ܱ������г���һ������Ga��NH3������Ӧ��ʵ���÷�Ӧ��ϵ���¶ȡ�ѹǿ�����������ͼ��ʾ��ͼ��A����C��Ļ�ѧƽ�ⳣ���ֱ�ΪKA��KC,���й�ϵ��ȷ����_________(�����)��

a������a��ʾNH3��ת���� b������a��ʾNH3��������� c��T1<T2 d��KA<Kc
������Ԫ�����ڱ�λ�ڵ������ڵڢ�A�壬��ѧ�����������ơ������������ȶ���������ˮ�����ܻ����ܽ����ȵ�NaOH��Һ�У��÷�Ӧ�����ӷ���ʽΪ_____________________��
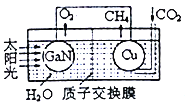
(3)�õ�������ͭ�����ͼ��ʾ���˹����ϵͳ�����ø�װ�óɹ�����CO2��H2OΪԭ�Ϻϳ�CH4��ͭ�缫���淢���ĵ缫��ӦʽΪ___________�����缫�ų�O2��CH4��ͬ�����µ������Ϊ________��Ϊ��߸��˹����ϵͳ�Ĺ���Ч�ʣ�����װ���м���������__________(����ᡱ�����ᡱ)��