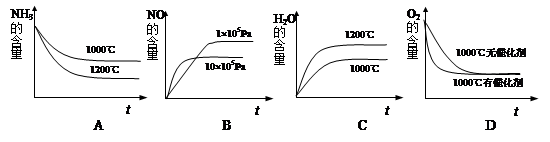��Ŀ����
��16�֣���������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3��s��+3 H2��g�� W��s��+3 H2O��g��
W��s��+3 H2O��g��
��ش��������⣺
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ
��2��ij�¶��·�Ӧ��ƽ��ʱ�����¶ȵ����ߣ� H2��ˮ����������ȼ�С����÷�ӦΪ_____________��Ӧ(�� ���Ȼ����)
��3�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ______ _________��,
��4����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
WO2��s��+2 H2��g�� W��s��+2H2O��g�� ��H��+66 kJ·mol-1
W��s��+2H2O��g�� ��H��+66 kJ·mol-1
WO2��g��+2 H2��g�� W��s��+2H2O��g�� ��H�� ��137.9 kJ·mol-1
W��s��+2H2O��g�� ��H�� ��137.9 kJ·mol-1
��WO2��s��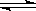 WO2��g���Ħ�H��_____ _______.
WO2��g���Ħ�H��_____ _______.
��5����˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��
����˵����ȷ����____________.
A���ƹ��ڵ�I2��ѭ��ʹ��
B��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
C��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
D���¶�����ʱ�� WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
WO3��s��+3 H2��g��
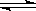 W��s��+3 H2O��g��
W��s��+3 H2O��g����ش��������⣺
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ
��2��ij�¶��·�Ӧ��ƽ��ʱ�����¶ȵ����ߣ� H2��ˮ����������ȼ�С����÷�ӦΪ_____________��Ӧ(�� ���Ȼ����)
��3�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25 �� ~ 550 �� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
��4����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
WO2��s��+2 H2��g��
 W��s��+2H2O��g�� ��H��+66 kJ·mol-1
W��s��+2H2O��g�� ��H��+66 kJ·mol-1WO2��g��+2 H2��g��
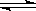 W��s��+2H2O��g�� ��H�� ��137.9 kJ·mol-1
W��s��+2H2O��g�� ��H�� ��137.9 kJ·mol-1��WO2��s��
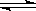 WO2��g���Ħ�H��_____ _______.
WO2��g���Ħ�H��_____ _______.��5����˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��
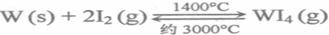
����˵����ȷ����____________.
A���ƹ��ڵ�I2��ѭ��ʹ��
B��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
C��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
D���¶�����ʱ�� WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
��K��c3(H2O)/c3(H2) ������ ��2WO3+H2 W2O5+H2O�� W2O5 , WO2
W2O5+H2O�� W2O5 , WO2
�� +203.9KJ/mol ��AB
 W2O5+H2O�� W2O5 , WO2
W2O5+H2O�� W2O5 , WO2�� +203.9KJ/mol ��AB
�����������1����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը��ݷ�Ӧ�Ļ�ѧ����ʽ��֪���÷�Ӧ��ƽ�ⳣ������ʽK��c3(H2O)/c3(H2)��
��2����Ӧ��ƽ��ʱ�����¶ȵ����ߣ� H2��ˮ����������ȼ�С����˵�������¶�ƽ������Ӧ�����ƶ�����������Ӧ�����ȷ�Ӧ��
��3�����ݱ������ݿ�֪����һ�ε���������W2O5�����Է�Ӧ�Ļ�ѧ����ʽ��2WO3��H2
 W2O5��H2O��ͬ�����ݱ��е����ݿ�֪��580��ʱ���������ʵ���Ҫ�ɷ�ΪW2O5�� WO2��
W2O5��H2O��ͬ�����ݱ��е����ݿ�֪��580��ʱ���������ʵ���Ҫ�ɷ�ΪW2O5�� WO2����4�����ݸ�˹���ɿ�֪���٣��ڼ��õ�WO2��s��
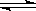 WO2��g�������Ԧ�H����66kJ/mol��137.9kJ/mol����203.9kJ/mol��
WO2��g�������Ԧ�H����66kJ/mol��137.9kJ/mol����203.9kJ/mol����5���÷�Ӧ�ڲ�ͬ�¶��£���Ӧ���еķ����Dz�ͬ�ġ����������ɵ��ʵ⣬���¶Ƚ��ͺ������ĵ⣬���Եƹ��ڵ�I2��ѭ��ʹ�ã�A��ȷ���÷���ʽ��֪��WI4�ڵ�˿�Ϸֽ⣬������W�Ǹ��⣬������ڵ�˿�ϣ�B��ȷ����C����ȷ�������¶ȣ���Ӧ���ʶ�������D����ȷ����ѡAB��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�������ӱ��ע�ؿ���ѧ���������⡢����������������������дƽ�ⳣ���ı���ʽʱ��Ҫ�ر���ע�����ʵ�״̬����ͬ����������ѧ�������Ӧ�������Լ��Ͻ�����˼ά������

��ϰ��ϵ�д�
�����Ŀ
 2C(g)����H��0��ƽ���ƶ���ϵ��ͼ��ʾ������˵����ȷ���ǣ��� ��
2C(g)����H��0��ƽ���ƶ���ϵ��ͼ��ʾ������˵����ȷ���ǣ��� ��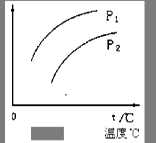
 2NH3(g)������Ӧ������n(H2)��n(NH3)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ���������й���������ȷ����
2NH3(g)������Ӧ������n(H2)��n(NH3)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ���������й���������ȷ����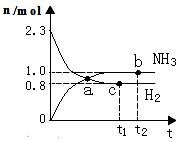

 CO2 + H2����830K�¶��´ﵽƽ�⡣
CO2 + H2����830K�¶��´ﵽƽ�⡣ 2HI(g)
2HI(g) 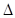 H��0�� �ﵽƽ����ı��������������Ũ�ȡ���ϵѹǿ�ȶ�����������Ŀ�ı仯ʱ������Ϊƽ��һ���������ƶ��ı�־���ǣ� ��
H��0�� �ﵽƽ����ı��������������Ũ�ȡ���ϵѹǿ�ȶ�����������Ŀ�ı仯ʱ������Ϊƽ��һ���������ƶ��ı�־���ǣ� �� 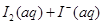

 ijI2����KI�����Һ�У�
ijI2����KI�����Һ�У� �����ʵ���Ũ��c(
�����ʵ���Ũ��c(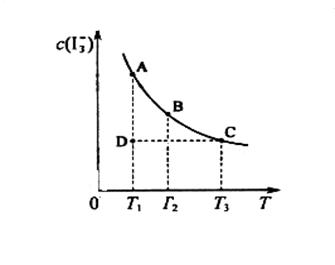
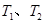 ����Ӧ��ƽ�ⳣ���ֱ�Ϊ
����Ӧ��ƽ�ⳣ���ֱ�Ϊ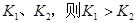
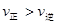
 CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±��� 4NO(g)+6H2O(g) ��H��0���˷�Ӧ��ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����
4NO(g)+6H2O(g) ��H��0���˷�Ӧ��ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����