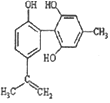
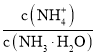��Ŀ����
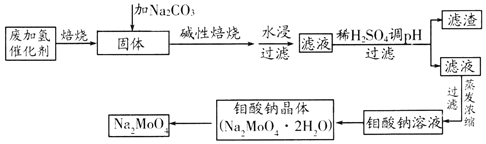
����Ŀ�������ƣ�Na2MoO4����һ����Ҫ�Ļ���ԭ�ϡ��÷ϼ������������MoS2��Al2O3��Fe2O3��SiO2�ȣ�Ϊԭ����ȡ�����ƣ�����������ͼ��ʾ��
��֪��MoO3��A12O3�ڸ������ܸ�Na2CO3������Ӧ��
�ش��������⣺
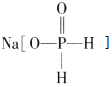
(1)Na2MoO4��MoԪ�صĻ��ϼ�______��
(2)�ϼ����������Ŀ�����ѳ�������֬����ȡ�����ݱ���ʵ�����ݷ������ϼ������Ԥ�����¶�Ӧѡ��______����
�ϴ����ڲ�ͬ�¶��µ��ղУ�ʱ�䣺2h��
�¶� /�� | 300 | 350 | 400 | 500 | 600 |
��ǰ /g | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
�պ� /g | 48.09 | 47.48 | 47.19 | 46.55 | 46.52 |
�ղУ� % | 96.2 | 95.0 | 94.4 | 93.1 | 93.0 |
(3)����ʱ����MoO3�Ļ�ѧ����ʽΪ______��������1mol MoO3ת�Ƶ�����Ϊ______NA��
(4)��̼���Ƽ��Ա���ʱ��Ҫ��Ӧ�Ļ�ѧ����ʽΪ______��
(5)��50t��MoS2Ϊ80%�ķϼ��������������ȡ�����롢�ᴿ���õ�30.9t Na2MoO4����Na2MoO4�IJ���Ϊ______��
���𰸡�+6 500 2MoS2+7O2![]() 2MoO3+4SO2 14 MoO3+Na2CO3
2MoO3+4SO2 14 MoO3+Na2CO3![]() Na2MoO4+CO2�� 60%
Na2MoO4+CO2�� 60%
��������
�������̣������б��շϼ��������MoS2ȼ�շ�Ӧ����ʽΪ2MoS2+7O2![]() 2MoO3+4SO2����Na2CO3���Ա��գ��ټ�ˮ�ܽ����������Һ��Ȼ������Һ�м���ϡ�������pHֵ�����ˣ���Һ�е�����ΪNa2MoO4�����Լ�̼���Ƽ��Ա���ʱ��Ҫ�ǽ�MoO3ת��Ϊ������ˮ��Na2MoO4����Ӧ�Ļ�ѧ����ʽΪMoO3+Na2CO3
2MoO3+4SO2����Na2CO3���Ա��գ��ټ�ˮ�ܽ����������Һ��Ȼ������Һ�м���ϡ�������pHֵ�����ˣ���Һ�е�����ΪNa2MoO4�����Լ�̼���Ƽ��Ա���ʱ��Ҫ�ǽ�MoO3ת��Ϊ������ˮ��Na2MoO4����Ӧ�Ļ�ѧ����ʽΪMoO3+Na2CO3![]() Na2MoO4+CO2��������Һ����Ũ������ȴ�ᾧ���õ�Na2MoO42H2O���壬�ٷֽ�õ�Na2MoO4���ݴ˷�������
Na2MoO4+CO2��������Һ����Ũ������ȴ�ᾧ���õ�Na2MoO42H2O���壬�ٷֽ�õ�Na2MoO4���ݴ˷�������
(1)����Na2MoO4�л��ϼ۴�����Ϊ0��MoԪ�صĻ��ϼ�Ϊ+��2��4-1��2��=+6�ۣ�
�ʴ�Ϊ��+6��
(2)�ɷϼ����������Ŀ�����ѳ�������֬����ȣ���ϱ���ʵ�����ݿ�֪��500�����ղеİٷֺ����������䣬���Էϼ������Ԥ�����¶�Ӧѡ��500�����ʴ�Ϊ��500��
(3)�����б��շϼ��������MoS2ȼ�շ�Ӧ����MoO3�Ļ�ѧ����ʽΪ2MoS2+7O2![]() 2MoO3+4SO2����Ӧ��MoԪ�ػ��ϼ���+4�����ߵ�+6��SԪ�ػ��ϼ���-2���ߵ�+4�����ɷ���ʽ��֪ת�Ƶ�����Ϊ2����6-4+2��6��=28e-�����Ե�����1mol MoO3ת�Ƶ�����Ϊ14NA��
2MoO3+4SO2����Ӧ��MoԪ�ػ��ϼ���+4�����ߵ�+6��SԪ�ػ��ϼ���-2���ߵ�+4�����ɷ���ʽ��֪ת�Ƶ�����Ϊ2����6-4+2��6��=28e-�����Ե�����1mol MoO3ת�Ƶ�����Ϊ14NA��
�ʴ�Ϊ��2MoS2+7O2![]() 2MoO3+4SO2��14��
2MoO3+4SO2��14��
(4)��̼���Ƽ��Ա���ʱ��Ҫ�ǽ�MoO3ת��Ϊ������ˮ��Na2MoO4����Ӧ�Ļ�ѧ����ʽΪMoO3+Na2CO3![]() Na2MoO4+CO2����
Na2MoO4+CO2����
�ʴ�Ϊ��MoO3+Na2CO![]() Na2MoO4+CO2����
Na2MoO4+CO2����
(5)��50t��MoS2Ϊ80%�ķϼ����������MoS2�����ʵ���Ϊ![]() =2.5��105mol������Moԭ���غ�n��Mo��=n��MoS2��=n��Na2MoO4��������Na2MoO4�IJ���=
=2.5��105mol������Moԭ���غ�n��Mo��=n��MoS2��=n��Na2MoO4��������Na2MoO4�IJ���=![]() ��100%=60%���ʴ�Ϊ��60%��
��100%=60%���ʴ�Ϊ��60%��

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
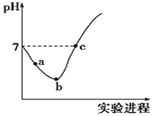
ѧϰʵ����ϵ�д�����Ŀ���뻯ѧƽ�����ƣ�����ƽ���ƽ�ⳣ�����������볣������K��ʾ�����±���ij�¶��¼��ֳ�������ĵ���ƽ�ⳣ����
�� | ���뷽��ʽ | ����ƽ�ⳣ��K |
CH3COOH | CH3COOH | 2��10-5 |
HClO | HClO | 3.0��10-8 |
H2CO3 | H2CO3 HCO3- | K1=4.4��10-7 K2=5.6��10-11 |
H3PO4 | H3PO4 H2PO4- HPO42- | K1=7.1��10-3 K2=6.3��10-8 K3=4.2��10-13 |
�ش��������⣺
��1������CH3COOH��HClO��H2CO3��HCO3-��H3PO4��H2PO4-��HPO42-���������ᣬ������������ǿ����___________���ѧʽ����
��2����NaClO��Һ��ͨ�������Ķ�����̼�����������ӷ���ʽΪ________��
��3�����¶���0.1mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ���������__��
A c��H+�� B c��H+��c��CH3COO![]()
��4��ȡ�������pH��Ϊa�Ĵ���ʹ���������Һ���ֱ��õ�Ũ�ȵ�NaOHϡ��Һǡ���кͣ����ĵ�NaOH��Һ������ֱ�ΪV1��V2�����С��ϵΪ��V1_________V2����������������������=������
��5�������������ӽ��H+������ǿ����________��
A HCO3- B CO32- C ClO- D CH3COO-
��6�������ʵ����Ŀ����Ʒֱ���pHΪ2��3�Ĵ�����Һ�кͣ������Ĵ�����Һ���������ΪVa��Vb�������ߵĹ�ϵ��ȷ����_________��
A Va��10Vb B Va��10Vb C Vb��10Va D Vb��10Va
��7����֪100��ʱ��ˮ�����ӻ�����Kw=1.0��10-12��pH=3��CH3COOH��pH=9��NaOH��Һ�������ϣ������Һ��_____________�ԣ�
��8����Ũ�ȵĢ٣�NH4��2SO4����NH4HSO4����NH4HCO3����NH4Cl����NH3H2O��Һ�У�NH4+Ũ���ɴ�С��˳���ǣ�_________��
��9��������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh_________��
��10�����ʵ���Ũ����ͬ��������Һ��a CH3COONa��b NaHCO3��c NaClO��d Na2CO3
������Һ��pH��С�������е�˳����____________���ñ����д����