��Ŀ����
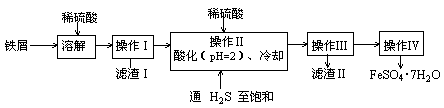
�̷����壨FeSO4��7H2O��M=278g/mol��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡�ʵ�����������᳧����������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�����Ʊ��̷��Ĺ������£�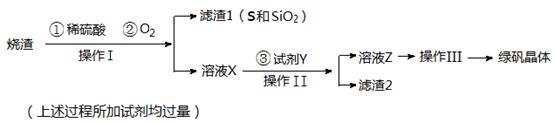 �Իش�
�Իش�
��1��������Ϊ ����д�������ƣ���
��2�� �Լ�Y����ҺX��Ӧ�����ӷ���ʽΪ ��
��3�����������̷������к���Fe2+��ʵ������� ��
��4���������˳������Ϊ�� ����ȴ�ᾧ������ �� �����
��5��ijͬѧ������KMnO4��Һ�ⶨ�̷���Ʒ��Fe2+������
a.��ȡ11.5g�̷���Ʒ���ܽ⣬���Ƴ�1000mL��Һ��
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٲ���a������Һʱ��Ҫ�IJ�������������������Ͳ���ձ�����ͷ�ι��⣬���� ��
�ڸ�ͬѧ��Ƶ����еζ���ʽ����������� (�гֲ�����ȥ)(����ĸ���)��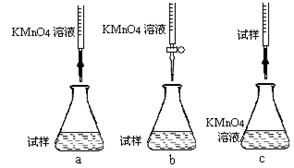
�۵ζ�ʱ������Ӧ�����ӷ���ʽΪ�� ��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����� �����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������KMnO4��ҺҺ�棬������������ȷ����ʹ�ⶨ��� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
�ݼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
��18�֣�
��1�����ˣ�1�֣� ��2�֣�
��2��Fe+2Fe3+= 3Fe2+ Fe+2H+=Fe2++H2������2�֣�
��3�����������þ������KSCN��Һ����ɫ��������Һ�м�����ˮ��ɺ�ɫ����2�֣��������������𰸣�
��4������Ũ����ϴ�ӣ���1�֣�
��5����1000mL����ƿ ��1�֣�
��b��1�֣�
�� 5Fe2++MnO4-+8H+=5Fe3++Mn2++4H20��2�֣�
�ܵμ����һ��KMnO4��Һʱ����Һ�����ɫ�Ұ���Ӳ�����ɫ��2�֣�ƫ�ͣ�1�֣�
��96.7%
���������������������H2SO4��O2��Ӧ���γ��˹������Һ�����Բ�����Ϊ���ˣ��𰸣����ˣ���YΪFe2(SO4)3����H2SO4��Ҫ��Fe������ת����FeSO4����ӦΪ��Fe+2Fe3+= 3Fe2+ Fe+2H+=Fe2++H2�����𰸣�Fe+2Fe3+= 3Fe2+ Fe+2H+=Fe2++H2����
�Ǽ��������̷������к���Fe2+��ʵ������ǣ��ȼ�����Һ����Fe3�� ��Ȼ���ٽ�Fe2�� ������Fe3�� ����KSCN��Һ���飬�𰸣����������þ������KSCN��Һ����ɫ��������Һ�м�����ˮ��ɺ�ɫ��
�ȴ�FeSO4��ˮ��Һ�л�þ��壺����Ũ�����ᾧ����������ϴ�ӣ�ϴ�ӡ�����𰸣�����Ũ����ϴ�ӣ�
�ɢ����Ƴ�1000mL��Һ������Ҫ��1000mL����ƿ���𰸣�1000mL����ƿ�������Ը�����ؾ���ǿ�����ԣ����Է�����Ƥ�ܣ�Ӧʢ������ʽ�ζ����ڣ�����������Һ�����ԣ�Ӧʢ������ʽ�ζ����ڣ���b����ʣ��ʴ�Ϊ��b�������Ը�����ؾ���ǿ�����ԣ���Fe2������ΪFe3��������ԭΪMn2����ͬʱ����ˮ����Ӧ���ӷ���ʽΪ5Fe2��+MnO4��+8H��=5Fe3��+Mn2��+4H2O���ʴ�Ϊ��5Fe2��+MnO4��+8H��=5Fe3��+Mn2��+4H2O���ܸ�ʵ����������ԭ�ζ����յ�ʱKMnO4��Һǡ�ù���һ�Σ���Һ�����Ϻ�ɫ��30���ڲ���ɫ������Ҫ���ָʾ�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������KMnO4��ҺҺ�棬��������������KMnO4��Һ�����ƫС������õ�Fe2�� �ĺ����ͣ��ʴ�Ϊ����Һ����ɫ��Ϊ�Ϻ�ɫ��30���ڲ���ɫ��ƫ�ͣ�
���������ữ��0.01000mol��L��1 KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL�����ݷ�Ӧ����ʽ��С���㣺
5Fe2��+MnO4��+8H���T5Fe3��+Mn2��+4H2O
5 1
n��Fe2���� 0.01000mol��L��1��0.0200L
����õ���n��Fe2����=0.001mol��
��1000mL��Һ�к�Fe2��=0.001mol��1000/25=0.04mol��
FeSO4��7H2O���ʵ���Ϊ0.04mol������=0.04mol��278g��mol��1=11.12g��
��������=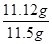 ��100%=96.70%��
��100%=96.70%��
�ʴ�Ϊ��96.70%��
���㣺�����������Ʊ�

 ȫ�ܲ��һ���þ�ϵ�д�
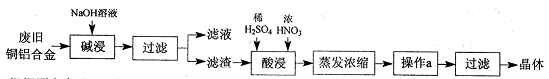
ȫ�ܲ��һ���þ�ϵ�д���ˮ��Ӧ��ǰ�������Ļ���ԭ����Դ,�Ӻ�ˮ�п���ȡ���ֻ���ԭ��.��ͼ�ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ����֪����XΪ��ⱥ��ʳ��ˮ���ã�ĸҺ��±����Ҫ����Ca2+��Mg2+,Cl-,SO42-��Br-������)��ش�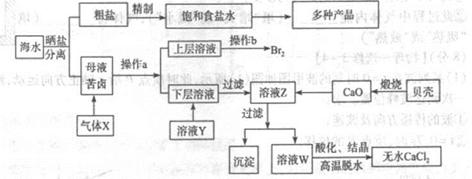
��1���ڴ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ���õ��Լ�Ϊ:
| A������ | B���Ȼ�����Һ | C������������Һ | D��̼������Һ��������Լ���˳����(����)______ |
��3��������ҺY��Ŀ����______����CaO������ҺZ��pH,���Գ�ȥMg2+�õ���ҺW���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ��______���ữ��ҺWʱ��ʹ�õ��Լ�Ϊ______

��4��������X����ͨ������װ������֤����X�����ʣ�
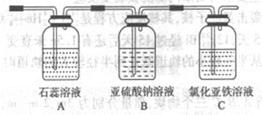
��ͨ������X��A�г��ֵ�������____________
��Cװ���з�����Ӧ�����ӷ���ʽΪ____________��
��������С��ͬѧ���һ��ʵ�飬֤��ϴ��ƿB�е�Na2SO3�ѱ�����(����ʵ�鲽�裩
________________________________________________________________________
��ȥ����������������������(������Ϊ����)����ѡ�õ��Լ��ͷ��뷽���ܴﵽʵ��Ŀ�ĵ���
| ѡ�� | ����� | �Լ� | ���뷽�� |
| A�� | �����飨�Ҵ��� | ��ʯ�� | ���� |
| B�� | ����(��ϩ�� | ���Ը��������Һ | ϴ�� |
| C�� | ������������ | ����ˮ | ��Һ |
| D�� | �����ᣨNaCl�� | ����ˮ | �ؽᾧ |
 N2+3Cu+3H2O�з�Ӧ��CuO��������H2O���������Բⶨͭ�Ľ������ԭ��������ʵ��װ�ã����ȼ��г�װ��δ���������¡�ʵ�鿪ʼʱ��Ӧ�ȵ�ȼ �����A������B�������ƾ��ƣ�c�м�ʯ�ҵ�����Ϊ ��
N2+3Cu+3H2O�з�Ӧ��CuO��������H2O���������Բⶨͭ�Ľ������ԭ��������ʵ��װ�ã����ȼ��г�װ��δ���������¡�ʵ�鿪ʼʱ��Ӧ�ȵ�ȼ �����A������B�������ƾ��ƣ�c�м�ʯ�ҵ�����Ϊ ��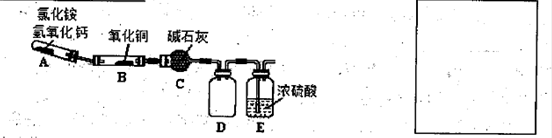
 ��Cl���ȣ�����ȡ�ϴ������Ȼ��ؾ��弰Һ�壬�������£�
��Cl���ȣ�����ȡ�ϴ������Ȼ��ؾ��弰Һ�壬�������£�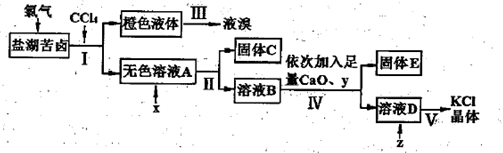
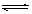 CH3COOC2H5+H20
CH3COOC2H5+H20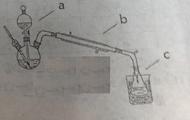



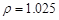 g/cm3 )����ȡ��36.5% (
g/cm3 )����ȡ��36.5% (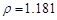 g/cm3 )������ mL
g/cm3 )������ mL