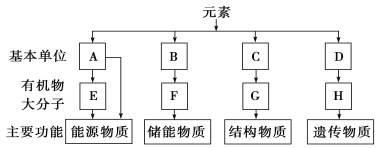
��Ŀ����
����Ŀ���ñ�Ũ�ȵ�NaOH��Һ���ζ�δ֪Ũ�ȵ����ᣬ���в����л�ʹ����ⶨŨ�ȱ�ʵ��Ũ��ƫ�ߵ��ǣ�������
�ټ�ʽ�ζ���������ˮϴ����δ�ñ���Һ��ϴ������ƿ������������ˮ��ʵ��ʱû�к�ɴ�������ȡδ֪Ũ���������ʽ�ζ���������ˮϴ����δ�ô���������ϴ���ܵζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ���ݵζ������ʱ�����Ӷ�����
A. �٢� B. �ڢ� C. �٢� D. �ܢ�
���𰸡�A
���������ټ�ʽ�ζ���������ˮϴ����δ�ñ���Һ��ϴ���ζ����ڼ�Һ��Ũ�Ⱦͻ��С�����Ի�������ļ ![]() ����ʹ
����ʹ![]() ƫ�ߣ�����ȷ��
ƫ�ߣ�����ȷ��
����ƿ������������ˮ��ʵ��ʱû�к�ɴ������������ڵζ����Ӧ����Ӱ�죬ԭ��������ƿ�м������ˮ����Ӱ��μӵ�����������Һ��������ڴ���
��ȡδ֪Ũ���������ʽ�ζ���������ˮϴ����δ�ô���������ϴ����ȡ�������ᱻˮϡ�ͣ�Ũ��ƫС�����µμӵ�����������Һ���ƫС�����յζ����ƫС���۴���
�ܵζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�����ʽ�ζ��ܵĶ��������μӽ���ƿ�������ԭ���ļ������ݵ���������Ե���![]() ����ʹ
����ʹ![]() ƫ�ߣ�����ȷ��
ƫ�ߣ�����ȷ��
�ݵζ������ʱ�����Ӷ����������ƫС�����Ե���![]() ��С��ʹ
��С��ʹ![]() ƫ�ͣ��ݴ���
ƫ�ͣ��ݴ���
����ƫ�ߵ��Ǣ٢ܣ�ѡ��A��ȷ��

����Ŀ������̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
(1)�õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ��
___ C+ ___ KMnO4+ H2SO4 �� ____CO2��+ ____MnSO4 + ____K2SO4+
(2)����ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)![]() CO2(g)��H2(g)���õ����¶������ݣ�
CO2(g)��H2(g)���õ����¶������ݣ�
ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
CO | H2O | H2 | CO | |||
1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
��ʵ��1����CO2��ʾ�Ļ�ѧ��Ӧ����Ϊ__________������С������λ������ͬ����
��ʵ��2������ƽ�ⳣ��K=_________���÷�ӦΪ _____��������š����ȷ�Ӧ��
(3)��֪�ڳ��³�ѹ�£�
�� 2CH3OH(l)��3O2(g) �� 2CO2(g)��4H2O(g) ��H �� ��1275.6 kJ��mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H �� ��566.0 kJ��mol
�� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д���״�����ȫȼ������һ����̼����̬ˮ���Ȼ�ѧ����ʽ��_____________��
(4)ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�á�

�ٸõ�ظ����ĵ缫��ӦΪ��_______________��
�ڸõ�ع���ʱ����Һ�е�OH����______���ƶ���
����Ŀ������ϸ������������ˮ����NO2��SO2�Ļ�ѧ��Ӧ�ǵ�ǰ�����ڼ������ε���Ҫ����·����ijʵ��С��������ijɷֽ�������֤�����ⶨ������SO2�ĺ�����̽��H2SO3�IJ������ʡ�
�ش�����������
��1���������ij��PM2.5��������Ҫ�ɷ֣��ռ�һ�������������������֤��
ȡһ�����������������Թ��У�����������ˮ�ܽ⣬����Һ�ֳ�����ʢ���Թ���:
�������� | ʵ������ | ���� |
��������һ��_______ | �а�ɫ�������� | ֤�����������к���SO42- |
������һ����_____�������Ӽг�ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ���Թ��������ݲ�����___________________ | ֤�����������к���NH4+���ۺ�����ʵ��,����˵���������ݿ����к���(NH4)2SO4 |
��2������ͼ��ʾ����װ�òⶨ������SO2�ĺ�����
���ⶨԭ����SO2ͨ���ĵ�����Һ�У�ʹ��Һ����ɫ��Ϊ��ɫ����Ӧ�Ļ�ѧ����ʽΪ______��
���ⶨ��������ij���㣬��ȡ5.0mL5.0��10-4mol/L�ĵ���Һ��ע��ͼ�е��Թ��У���2-3�ε���ָʾ������ʱ��Һ����ɫ����ͼ��װ�����Ӻ�����������ֹˮ�п��ƣ����г�����ȡ��ע�����������ظ�����ֱ����Һ����ɫȫ���ʾ�Ϊֹ������ȡ����8000.0mL�����øü��������SO2�ĺ���Ϊ_____mg/L��
��3��̽��H2SO3�IJ������ʡ�
ѡ�������װ�ú�ҩƷ̽��H2SO3��HClO������ǿ��
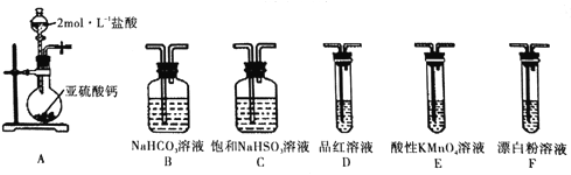
�ټ�ͬѧ��Ϊ����A��C��F��β��������˳������װ�ã�����֤��H2SO3��HClO������ǿ������ͬѧ��Ϊ�÷�������������������_________________��
�ڱ�ͬѧ���ü�ӷ�֤����ʵ�鷽��Ϊ������A��C____(����ĸ) ��β������˳������װ�ã�����װ��C��������___________��֤��H2SO3������ǿ��HClO�����Ե�ʵ��������_____��