��Ŀ����
����Ŀ����25��ʱ����1.0Lw mol��L-lCH3COOH��Һ��0.1molNaOH�����ϣ���ַ�Ӧ��Ȼ������Һ�м���CH3COOH��CH3COONa����������������¶ȱ仯����������ҺpH�ı仯��ͼ��ʾ������������ȷ����
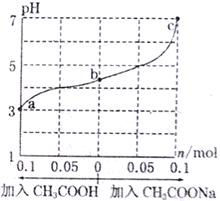
A. a��b��c��Ӧ�Ļ��Һ�У�ˮ�ĵ���̶��ɴ�С��˳����c>a>b
B. b����Һ��c(Na+)>c(CH3COO-)
C. ����CH3COOH������![]() ����
����
D. 25��ʱ��CH3COOH�ĵ���ƽ�ⳣ��Ka=![]()
���𰸡�D
�������������������25��ʱ����1.0Lw mol��L-lCH3COOH��Һ��0.1molNaOH�����ϣ���ַ�Ӧ����ͼ��֪���û��Һ�����ԣ�˵���û��Һ�Ǵ���������ƵĻ��Һ��c(CH3COONa)=0.1mol��L-l��![]() =
=![]() mol��L-l��A. ��Ϊ�����������ˮ�ĵ��룬�������ƴٽ�ˮ�ĵ��룬����a��b��c��Ӧ�Ļ��Һ�У�ˮ�ĵ���̶��ɴ�С��˳����c >b>a��A����ȷ��B. ��Ϊ��������Һ�Լ��ԡ�b����Һ�����ԣ�c(OH-)��c(H+)��˵��b����Һ�Ǵ���ʹ����ƵĻ��Һ���ɵ���غ��֪��c(Na+)��c(CH3COO-)��B����ȷ��C. ����CH3COOH�����У���Һ��pH��С����c(H+)������c(OH-)��С��
mol��L-l��A. ��Ϊ�����������ˮ�ĵ��룬�������ƴٽ�ˮ�ĵ��룬����a��b��c��Ӧ�Ļ��Һ�У�ˮ�ĵ���̶��ɴ�С��˳����c >b>a��A����ȷ��B. ��Ϊ��������Һ�Լ��ԡ�b����Һ�����ԣ�c(OH-)��c(H+)��˵��b����Һ�Ǵ���ʹ����ƵĻ��Һ���ɵ���غ��֪��c(Na+)��c(CH3COO-)��B����ȷ��C. ����CH3COOH�����У���Һ��pH��С����c(H+)������c(OH-)��С��![]() �������
�������![]() ���䣬���ԣ�
���䣬���ԣ�![]() ��С��C����ȷ��D. ��c���֪��pH=7ʱ��c(OH-)=c(H+)=
��С��C����ȷ��D. ��c���֪��pH=7ʱ��c(OH-)=c(H+)=![]() mol��L-l���ɵ���غ��֪����ʱc(Na+)=c(CH3COO-)=0.1mol��L-l+0.1mol��L-l=0.2mol��L-l��
mol��L-l���ɵ���غ��֪����ʱc(Na+)=c(CH3COO-)=0.1mol��L-l+0.1mol��L-l=0.2mol��L-l��![]() =
=![]() mol��L-l�����ԣ�25��ʱ��CH3COOH�ĵ���ƽ�ⳣ��Ka=
mol��L-l�����ԣ�25��ʱ��CH3COOH�ĵ���ƽ�ⳣ��Ka=![]() ��D��ȷ������ѡD��
��D��ȷ������ѡD��

 һ����������ϵ�д�
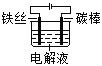
һ����������ϵ�д�����Ŀ����������(POCl3)����Ҫ�Ļ�������ԭ�ϣ��㷺������ҩ��Ⱦ�����ܽ���������ҵ��ij��ȤС��ģ��PCl3ֱ���������Ʊ�POCl3,ʵ��װ���������:
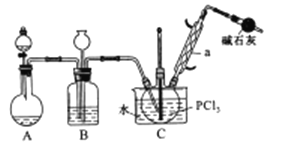
�й����ʵIJ����������±�:
�۵�/�� | �е�/�� | ���� | |
PCl3 | -112 | 75.5 | ��ˮ����H3PO3��HCl����O2����POCl3 |
POCl3 | 2 | 105.3 | ��ˮ����H3PO4��HCl,������PCl3 |
�ش���������:
(1)����a��������_____��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
(2)Bװ�õ����ó��۲�O2������֮�⡣����____________��
(3)Cװ�ÿ��Ʒ�Ӧ��60�桫65����У�����ҪĿ����____________��
(4)ͨ��������·����Բⶨ�������ײ�Ʒ��ClԪ�غ�����ʵ�鲽������:
I.ȡxg��Ʒ����ƿ�У���������NaOH��Һ������ȫ��Ӧ���ϡ���������ԡ�
II.����ƿ�м���0.1000mol/L��AgNO3��Һ40.00mL,ʹCl-��ȫ������
III.�����м���2mL������������ҡ����ʹ�������汻�л��︲�ǡ�
IV.����ָʾ������cmol/LNH4SCN��Һ�ζ�����Ag+���յ㣬�����������VmL��
��֪:Ksp(AgCl)=3.2��10-10,Ksp(AgSCN)=2��10-12
�ٵζ�ѡ�õ�ָʾ����_______(����)���ζ��K�������Ϊ_____________��
a.FeCl2 b.NH4Fe(SO4)2 c.���� d.����
��C1Ԫ�ص������ٷֺ���Ϊ(�г���ʽ)____________��
�۲���III������������Ŀ����___�����˲���������C1Ԫ�غ�������___(�ƫ��ƫС�����䡱)��
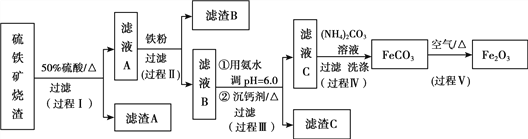
����Ŀ��ij����������������������Fe2O3��Fe3O4��Al2O3��CaO��SiO2�ȣ�Ϊԭ����ȡ������Fe2O3��Ҫ��>99.2%��CaO����<0.01%�����乤����������(�������Լ����Թ���)��
��֪���������������pH
Al(OH)3 | Fe(OH)2 | Fe(OH)3 | |
��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
��1������A����Ҫ�ɷ���__________��
��2���ڹ������пɹ۲쵽�����������ݣ���Һ��ɫ������dz���ܽ���ʵ����������ӷ���ʽ��__________����Ӧ������__________��Һ����˵��Fe3+�Ƿ��Ѿ���ȫ��Ӧ��
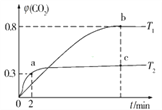
��3���ڹ������У����������ҺAϡ�Ͳ�ͬ������,����������Ĺ�������,�ó�Fe3+Ũ�ȡ���ԭ�ʺͷ�Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
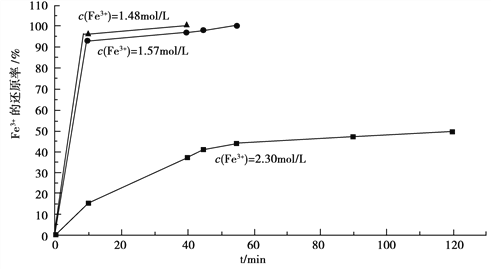
�������ʵ����˵����������ѡ��ϡ�ͺ�c(Fe3+)Ϊ1.60mol/L���ҵ�������______��
��4���ڹ������У�����������ͬ�����£���ѡ���˲�ͬ���Ƽ�����ʵ�飬ʵ�����ݼ��±���
����֪����ҺB�иƵĺ�����CaO��Ϊ290��310mg/L��
���Ƽ� | Na2SO3 | H2C2O4 | (NH4)2CO3 | Na2CO3 | NH4F |
����/g | 2 | 2 | 2 | 5 | 2 |
ʣ��CaO/mg/L) | 290 | 297 | 290 | 190 | 42 |
����ʵ������ѡ�����˵ij��Ƽ����õ�����C����Ҫ�ɷ���__________��
��5���ڹ������У���Ӧ�¶���Ҫ������35�����£����˹��ߣ�����ܵ�ԭ����__________��
��6���ڹ������У���Ӧ�Ļ�ѧ����ʽ��__________��
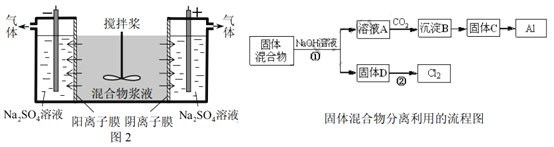
����Ŀ��ij��ѧС������Ϊ������̽����ͬ���������������ĵ缫��Ӧ��
ʵ��װ�� | ʵ�� | ��ѹ | ���Һ | ʵ������ |
| �� | 3V | 3mol/LNa2SO4��Һ | ̼�������д������ݲ�������Һ��ƣ��к��ɫ�������� |
�� | 3V | 3 mol/L KI��Һ | ̼�������д������ݲ�������˿������Һ��Ϊ��ɫ����Ϊ�غ�ɫ��Լ5min���غ�ɫ��ʧ����������ɫ��״�� | |
�� | 3V | 3mol/L NaOH��Һ | ���缫�϶��д������ݲ�����5min��ֹͣʵ�飬ȡ����˿����˿�����ܸ������к���ɫ���壬��Һ��δ������� |
��1����ʵ������̼�����������������__________��
��ʵ��������˿����������ĵ缫��ӦʽΪ__________��
����ʵ�����У�Ϊ��֤��˿�缫�IJ��ȡ������˿������ɫ��Һ���Թ��У��μ�2��K3Fe(CN)6��Һ�������Ա仯����ȡ������˿������ɫ��Һ���鷢����Һ����I2�����鷽����__________���Ա�ʵ���������������ɵó��Ľ�����__________��
��2��Ϊ��һ��̽������c(OH-)��������Ӧ��Ӱ�죬��С������ԭװ������ʵ������
ʵ�� | ��ѹ | ���Һ | ʵ������ |
�� | 3V | 10 mol/L NaOH��Һ | ���缫�϶��д������ݲ�������̼���ϵ�����Զ������˿������������Һ���Ϻ�ɫ��ֹͣʵ�飬��˿���Ա�ϸ�����Һ��Ȼ���� |
�������ϣ�FeO42-����Һ�г��Ϻ�ɫ�������缫��Ӧʽ��__________��
��3��Ϊ̽��ʵ��������Һ��Ƶ�ԭ��С������ԭװ������ʵ����������
ʵ�� | ��ѹ | ���Һ | ʵ������ |
�� | 3V | �����ȴ��3 mol/L Na2SO4��Һ | ̼�������д������ݲ�������˿��Χ����һ���ɫ��״������2min������Ϊ��ɫ�� |
�� | 8V | �����ȴ��3 mol/L Na2SO4��Һ | ̼������Ѹ�ٲ����������ݣ���˿���������ݣ�1min����Χ����һ���ɫ��״������2min����̣���Һ���к��ɫ����� |
��ʵ�����а�ɫ��״������Ϊ���ɫ�ķ�Ӧ��ѧ����ʽ��__________��
���ɴ�ȷ��ʵ��������Һ��Ƶ�ԭ����__________��
��4���ۺ�����ʵ�飬����Ϊ����ʱ��Ӱ��������е������缫�����������__________��