��Ŀ����
����Ŀ����Ԫ���ڵ����Ϻ����ḻ�����а����ڹ�ũҵ����������������Ҫ�����á�
��1�������е���_________���������ţ���ʽ���ڡ��̵��ǵ�ѭ������Ҫ���ڣ���ҵ�̵���NH3��Ӧ�¶�ѡ��500�����ҵ�ԭ����________________________________��
��2��ij��ȤС������ͼװ��̽�����Ĵ����������Ȳ�����2һ��ʱ���ѹ1�д��������������۲쵽2�����ʳʺ���״̬��ֹͣ���Ⱥ����ܱ��ֺ��ȣ��÷�Ӧ��_________��Ӧ(��������������������)��
��3����ͼ����ȡ��3����4�н��۲쵽�������̣��ð��̵Ļ�ѧʽ����Ϊ______________��
��4��ʵ���һ�����Ũ��ˮ���������ƹ����ϣ��Ƶð���������ƽ���ƶ���������ص����۽��÷������Ʊ�������ԭ��_____________________________________________��
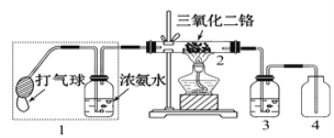
��5������ͼװ�ý��а���������ʵ�飨����1������2ʵ��ǰ�رգ���G��H��������������������ȴ�����1��һ��ʱ��ر�����1���ٴ�����2��Hƿ��������������________________________��

���𰸡� N2 �������������¶ȡ�ͬʱ������500C�ӿ췴Ӧ���� ���� NH4NO3 ��֪��ˮ�д��ڣ�NH3+H2O![]() NH3H2O
NH3H2O![]() NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ� ������1�а��̣�������2��ʯ����Һ������Hƿ��ʯ����Һ����˺�ɫ
NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ� ������1�а��̣�������2��ʯ����Һ������Hƿ��ʯ����Һ����˺�ɫ
����������������������������Ʊ�Ϊ��ģ���Ҫ���������ɡ���Ӧ������ѡ����������ѧ��Ӧ����ѧƽ���ƶ�ԭ������Ȫʵ��ԭ���Ȼ���֪ʶ�����鿼���Ի���֪ʶ�����������ͷ�����������������
��������1�������к��еĵ�Ԫ����Ҫ��N2����ҵ�̵���ȡNH3�ķ�Ӧԭ��Ϊ��N2+3H2![]() 2NH3���÷�ӦΪ���ȷ�Ӧ�����¿�����߷�Ӧ���ʣ����Ƕ�ƽ����ϵ��NH3���ʲ��������ǵ�������500�����һ�����ǿ������ѡ���¶�Ϊ500�����ҡ���ȷ�𰸣�N2�� �������������¶ȡ�ͬʱ������500C�ӿ췴Ӧ��������2��ʵ�������ֹͣ���ȣ�����װ����Ȼɢ�ȣ��¶�ԭ��Ӧ�����ͣ�����ʵ��ʵ����װ��2��Ȼ�ܱ��ֺ��ȣ�˵���÷�Ӧ�����ͷ���������ȷ�𰸣����ȡ���3����2ʵ��װ�ã�װ��2�����NH3��O2���ַ�Ӧ����NO��H2O��ԭװ��3��ȥû�з�Ӧ��NH3�����ȡ��װ��3������2NO+O2=2NO2��3NO2+H2O=2HNO3+NO��HNO3+NH3=NH4NO3���������ɵİ�����NH4NO3����ȷ�𰸣�NH4NO3����4����֪��ˮ�д��ڣ�NH3+H2O
2NH3���÷�ӦΪ���ȷ�Ӧ�����¿�����߷�Ӧ���ʣ����Ƕ�ƽ����ϵ��NH3���ʲ��������ǵ�������500�����һ�����ǿ������ѡ���¶�Ϊ500�����ҡ���ȷ�𰸣�N2�� �������������¶ȡ�ͬʱ������500C�ӿ췴Ӧ��������2��ʵ�������ֹͣ���ȣ�����װ����Ȼɢ�ȣ��¶�ԭ��Ӧ�����ͣ�����ʵ��ʵ����װ��2��Ȼ�ܱ��ֺ��ȣ�˵���÷�Ӧ�����ͷ���������ȷ�𰸣����ȡ���3����2ʵ��װ�ã�װ��2�����NH3��O2���ַ�Ӧ����NO��H2O��ԭװ��3��ȥû�з�Ӧ��NH3�����ȡ��װ��3������2NO+O2=2NO2��3NO2+H2O=2HNO3+NO��HNO3+NH3=NH4NO3���������ɵİ�����NH4NO3����ȷ�𰸣�NH4NO3����4����֪��ˮ�д��ڣ�NH3+H2O![]() NH3H2O
NH3H2O![]() NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ��¶������ܹ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ�����ȷ�𰸣���֪��ˮ�д��ڣ�NH3+H2O
NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ��¶������ܹ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ�����ȷ�𰸣���֪��ˮ�д��ڣ�NH3+H2O![]() NH3H2O
NH3H2O![]() NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ�����5��װ��ͼ���������HCl����NH3�������NH4Cl�������ܹ������а��̲�����������Ӧ���������������С������һ��ʱ��ر�����1���ٴ�����2���ձ���ʯ��ˮ��Һ�ᱻ��������Hƿ��HƿNH3��ˮ��������OH-�������Һ��ɫ��졣��ȷ�𰸣�������1�а��̣�������2��ʯ����Һ������Hƿ��ʯ����Һ����˺�ɫ��
NH4+ + OH�C���������ƹ�������ˮ����ˮ���Ĺ����Ƿ��ȵģ���ʹNH3H2O�ֽ�ų���������ˮ���������ƻ�Ϻ�����������������Һ�е����������ӵ�Ũ�ȣ�ʹ��ˮ����ƽ�������ƶ��������ڰ����ݳ�����5��װ��ͼ���������HCl����NH3�������NH4Cl�������ܹ������а��̲�����������Ӧ���������������С������һ��ʱ��ر�����1���ٴ�����2���ձ���ʯ��ˮ��Һ�ᱻ��������Hƿ��HƿNH3��ˮ��������OH-�������Һ��ɫ��졣��ȷ�𰸣�������1�а��̣�������2��ʯ����Һ������Hƿ��ʯ����Һ����˺�ɫ��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
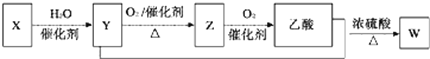
�����ܿ����ϵ�д�����Ŀ����X��Y��Z��W���ֶ���������Ԫ�أ�ԭ��������������Ԫ��������ԭ�ӣ���������ṹ���±���ʾ��
Ԫ�ر�� | Ԫ��������ԭ�ӣ���������ṹ |
X | ԭ�Ӻ���û������ |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Z | ���ڲ��������������2�� |
W | ������Ԫ����ԭ�Ӱ뾶��С |
��ش�
��1��д��Ԫ��Z�����ڱ��е�λ��______������Ԫ��W��ԭ�ӽṹʾ��ͼ______��
��2��Y��W��ȣ���̬�⻯���ȶ��Խ�������________���ѧʽ����ͬ������Ԫ������������Ӧˮ�������Ը�ǿ����__________��
��3��X��Y��Z����Ԫ�ؿ����γɻ�����ZYX2�����뻯����XW������Ӧ�����������Σ�д���÷�Ӧ�Ļ�ѧ����ʽ________________��
����Ŀ���������Ļ�����������������������Ҫ����;��NH3��HNQ3������Ҫ������Ʒ��
��1���ϳɰ���ԭ����N2��H2ͨ�����Խ�̿��ˮ�Ϳ���Ϊԭ������ȡ�ġ�����Ҫ��Ӧ�ǣ�
��2C+ O2��2CO
��C+H2O(g)��CO+H2
��CO+H2O(g)��CO2+H2
ij�������н���̿��H2O(g)�Ϳ������������N2��O2�������Ϊ4:1����ͬ����Ϸ�Ӧ������������ᆳ������������±�������x��_______m3��ʵ��������_____kg��̿��
���� | CO | N2 | CO2 | H2 | O2 |
�����m3������״���� | x | 20 | 12 | 60 | 1.0 |
��2��һ�������£�ͨ�����з�Ӧ��ʵ��ȼú��������Ļ���:2CO(g)+SO2(g)![]() 2CO2(g)+S(1) ��H<0������2L �����ܱ�������ͨ��2molCO��1molSO2,��Ӧ�ڲ�ͬ�����½���������Ӧ����Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ
2CO2(g)+S(1) ��H<0������2L �����ܱ�������ͨ��2molCO��1molSO2,��Ӧ�ڲ�ͬ�����½���������Ӧ����Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ

����ʵ��a��ȣ�c��ı��ʵ������������____________��
����P0��ʾ��ʼʱ��ѹǿ��P��ʾƽ��ʱ��ѹǿ��.������ʾSO2��ƽ��ת���ʣ���������ʽΪ________��
��3����֪N2O4![]() 2NO2��N2O4��NO2������¶���264-413K,�����ܵ�264Kʱ��ȫ��Ϊ��ɫ��N2O4 ���壬�ﵽ264KʱN2O4��ʼ�ֽ⣬�е�294Kʱ��Ϊ����ɫ�Ļ�����壬�¶ȸ���413Kʱ�������ֱ�Ϊ��ɫ����2NO2
2NO2��N2O4��NO2������¶���264-413K,�����ܵ�264Kʱ��ȫ��Ϊ��ɫ��N2O4 ���壬�ﵽ264KʱN2O4��ʼ�ֽ⣬�е�294Kʱ��Ϊ����ɫ�Ļ�����壬�¶ȸ���413Kʱ�������ֱ�Ϊ��ɫ����2NO2![]() 2NO+O2 ������1L���ܱ������з�����ӦN2O4
2NO+O2 ������1L���ܱ������з�����ӦN2O4![]() 2NO2�ﵽƽ��״̬��
2NO2�ﵽƽ��״̬��
������ʱΪ��״̬��(273K 101KPa)���������м���4.6g����NO2����ﵽƽ��ʱ��������ɫ____(��ԭƽ��״̬�ȣ���ѡ����ĸ����ͬ)
A.����(����) B.��С(��dz) C.���� D.����ȷ��
������ʱΪ25����101KPa�£��������м���4.6g����NO2����ﵽƽ��ʱ��������ɫ______���������NO2���������___________��
��4���������Ͽ�֪�������£�Ksp[Ag(NH3)2-]= 1.00��107��Ksp[AgC1]=2.50��10-10.
��������Һ�д���ƽ�⣺Ag+(aq)+2NH3(aq)![]() Ag(NH3)2+(aq)���÷�Ӧƽ�ⳣ���ı���ʽΪK��=__________��
Ag(NH3)2+(aq)���÷�Ӧƽ�ⳣ���ı���ʽΪK��=__________��
�ڼ���õ����淴ӦAgCl (s)+2NH3(aq)![]() Ag(NH3)2+(aq)+Cl��(aq)�Ļ�ѧƽ�ⳣ��K=______��1 L 1 mol/L��ˮ���������ܽ�AgCl_______mol������2λ��Ч���֣�
Ag(NH3)2+(aq)+Cl��(aq)�Ļ�ѧƽ�ⳣ��K=______��1 L 1 mol/L��ˮ���������ܽ�AgCl_______mol������2λ��Ч���֣�