��Ŀ����
����Ŀ��ʵ�����Ʊ������飨C2H5Br���ķ���Ϊ��
NaBr �� H2SO4 �� NaHSO4 �� HBr
C2H5OH �� HBr ![]() C2H5Br �� H2O
C2H5Br �� H2O
���п��ܻᷢ������Ӧ:
2HBr �� H2SO4(Ũ) �� Br2 ���� SO2�� �� 2H2O
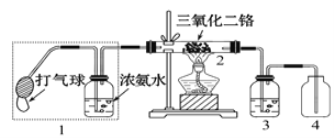
��ȡ��װ�úͲ�����ͼ����֪������ķе�38��4�棬������ˮ����
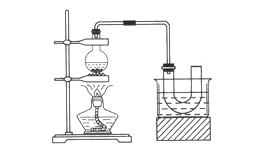
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ιܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10 mL 95���Ҵ���28 mL 78��Ũ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С�ļ��ȣ�ʹ���ַ�Ӧ��
�������Ϣ�ش��������⣺
��1����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ������������������ʽΪ___________��ͬʱ���ɵ���ɫ�������ʽΪ________________��
��2��Ϊ�˸��õĿ����¶ȣ�����ͼʾ��С������⣬���õļ��ȷ�ʽΪ________��
��3����Ӧ������U�ι��ڴ��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ�����ѡ�������Լ��е�________________________������ţ���
A��NaOH��Һ B��H2O C��Na2SO3��Һ D��CCl4
Ҫ��һ���Ƶô�����C2H5Br������ˮϴ��Ȼ�������ˮCaCl2���ٽ���__________����������ƣ���
��4�����м���ʵ�鲽�裬Ϊ�˼�������������Ԫ�أ�ȡ�������������Ʒ��������ȷ�IJ���˳���ǣ�___________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOHˮ��Һ������ȴ
A. �ܢ٢ݢۢ� B. �ܢۢ٢ݢ� C. �ܢۢڢ٢� D. �ܢڢ٢ۢ�
���𰸡� Br2 SO2 ˮԡ���� C ��Һ A
������������ʵ�鷽����������ۣ���1���¶ȹ��ߣ�Ũ�������ǿ�����ԣ��ܰ�Br��������Br2����˺���ɫ����ΪBr2��ͬʱH2SO4����ԭ��SO2����ɫ����ΪSO2����2��Ϊ�˵õ�����ȶ����¶ȣ���Ҫˮԡ���ȣ���3���ֲ�Ʒ�л����Ҵ���Һ�壬��ȥ��Щ���ʣ�A��C2H5Br����NaOH����ˮ�ⷴӦ����˲���ʹ��NaOH��ȥ���ʣ���A����B��Һ����ˮ�е��ܽ�Ƚ�С������Һ��Ч�������룬��B����C�����������Ҵ���ˮ����������ܣ��Լ�Һ�����ǿ�����ԣ��ܰ�Na2SO3������Na2SO4����������ԭ������ˮ��Br������C��ȷ��D��CCl4�����л��ܼ���C2H5Br����CCl4�������µ����ʣ���D������ˮϴ��Ŀ���dz�ȥ����ˮ�����ʣ�������ˮCaCl2��Ŀ���ǽ��и����ΪC2H5BrΪҺ�壬��˲��÷�Һ�ķ������з��룻��4����������������Ԫ�أ��Ȱ���Ԫ��ת���Br���������������������Һ�������ȣ�Ȼ����ȴ�����������ữ���ټ�����������Һ�����ֵ���ɫ������˵��������Ԫ�أ���֮�����У�˳���Ǣܢ٢ݢۢڣ���ѡ��A��ȷ��