��Ŀ����
����Ŀ��(1)��11.2 g KOH��ϡ��Һ��1 L0.1mol/L��H2SO4��Һ��Ӧ�ų�11.46 kJ���������÷�Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽΪ___________________��
(2)��0.40 mol N2O4�������2 L�ĺ����ܱ������з������·�Ӧ��N2O4(g) ![]() 2NO2(g)��H����T1���T2��ʱ�����NO2�����ʵ�����ʱ��仯��ͼ��ʾ��
2NO2(g)��H����T1���T2��ʱ�����NO2�����ʵ�����ʱ��仯��ͼ��ʾ��
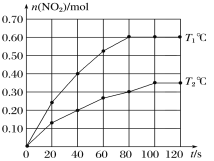
��T1�棬40��80 s����N2O4��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ________mol/(L��s)��
����H________0(����>������<������=��)��
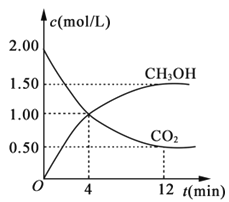
(3)��ס��������ݻ���Ϊ1 L�ĺ��������У��ֱ����2 mol A��2 mol B��1 mol A��1 mol B����ͬ������(�¶�T ��)���������з�Ӧ��A(g)+B(g)![]() xC(g) ��H<0�������������c(A)��ʱ��t�ı仯��ͼ��ʾ:
xC(g) ��H<0�������������c(A)��ʱ��t�ı仯��ͼ��ʾ:
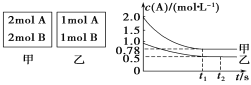
�ټ�����ƽ�������B��ת����Ϊ_______��
��T�� ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ________��
(4)��25 ���£���a mol/L�İ�ˮ��0.01mol/L������������ϣ���Ӧƽ��ʱ��Һ��c(NH4+)=c(Cl-)��
������Һ��________��(����������������������)��
���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��__________________��
���𰸡�KOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)=![]() K2SO4(aq)+H2O(l)����H=��57.3kJ/mol 0.00125 > 61% 2 ��
K2SO4(aq)+H2O(l)����H=��57.3kJ/mol 0.00125 > 61% 2 �� ![]()
��������
(1)�ȼ���KOH��H2SO4�����ʵ�����Ȼ���ж��������ʹ�����ȷ����Ӧ����1molҺ̬ˮ�ų������������ø÷�Ӧ���к��ȵ��Ȼ�ѧ����ʽ��
(2)�� Tl��ʱ��40s��80s�ڶ������������ʵ�����0.40mol��Ϊ0.60mol������v=![]() ������ö���������ʾ��ƽ����Ӧ���ʣ�Ȼ����ݷ�Ӧ�����뻯ѧ�����������ȼ������N2O4��ʾ��ƽ����Ӧ���ʣ�
������ö���������ʾ��ƽ����Ӧ���ʣ�Ȼ����ݷ�Ӧ�����뻯ѧ�����������ȼ������N2O4��ʾ��ƽ����Ӧ���ʣ�
�ڸ���ͼ�����߱仯��֪��Tl��ʱ��Ӧ���ʴ���T2�棬��T2��ﵽƽ��ʱ�������������ʵ���С��Tl�棬�����¶ȶԻ�ѧƽ���Ӱ���жϸ÷�Ӧ�ķ�Ӧ�ȣ�
(3)���ȸ���ͼʾ�������������A��ת�������ٽ�Ϸ���ʽ��A��B��ת����ϵ�������B��ת������������ת���ʵ���ת�����������ıȵõ������ʵ�ת���ʣ�
�ڼ���ס���������A��ת���ʣ��ٸ���ѹǿ��Aת���ʵ�Ӱ����ȷ��xֵ��������������ʽ�õ��÷�Ӧƽ��ʱ�����ʵ���Ũ�ȣ�����ƽ�ⳣ������ʽ���ɼ������ƽ�ⳣ����ֵ��
(4)��ˮ�����ᷢ����ӦHCl+NH3H2O�TNH4Cl+H2O�����ݵ���غ������Һ������ԣ��ٸ���ƽ��Ũ�ȼ��㰱ˮ�ĵ��볣����
(1)11.2g��0.2molKOH��ϡ��Һ��1L��0.1mol/L��H2SO4��Һ��Ӧ�ų�11.46kJ�����������м�е�OH-�����ʵ�����0.2mol������Һ���е�H+�����ʵ���Ϊ0.1mol/L��1L��2=0.2mol������ǡ�÷�Ӧ����0.2molˮ���ų�11.46kJ��������Ӧ����1molˮ�ų�����Ϊ11.46kJ��0.2=57.3kJ����˸÷�Ӧ�ı�ʾ�к��ȵ��Ȼ�ѧ����ʽΪ��KOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)=![]() K2SO4(aq)+H2O(l)����H=��57.3kJ/mol��
K2SO4(aq)+H2O(l)����H=��57.3kJ/mol��
(2)��Tl��ʱ��40s��80s�ڶ������������ʵ�����0.40mol��Ϊ0.60mol�����ö���������ʾ��ʱ��ε�ƽ����Ӧ����Ϊ��v(NO2)=![]() =0.0025mol/��Ls������ѧ��Ӧ�����뻯ѧ�����������ȣ���v(N2O4)=
=0.0025mol/��Ls������ѧ��Ӧ�����뻯ѧ�����������ȣ���v(N2O4)=![]() v(NO2)=0.00125mol/��Ls����
v(NO2)=0.00125mol/��Ls����
�ڸ���ͼ�����߱仯��֪��Tl��ʱ��Ӧ���ʴ���T2�棬���¶ȴ�СΪ��Tl�棾T2�棬����T2��ﵽƽ��ʱ�������������ʵ���С��Tl�棬˵�������¶ȣ�ƽ��������Ӧ�����ƶ�����÷�ӦΪ���ȷ�Ӧ����H��0��
(3)�ټ�������A��Ӧ�����ʵ���Ϊ��n(A)=(2-0.78)mol/L��1L=1.22mol������A��B�Ĺ�ϵʽ֪���μӷ�Ӧ��B�����ʵ���Ҳ��1.22mol����B��ת����=![]() ��100%=61%��
��100%=61%��
�ڼ�������Ӧ�ﵽƽ���c(A)0.78mol/L��A��ת����=![]() ��100%=61%���������У�A��ת����=
��100%=61%���������У�A��ת����=![]() ��100%=50%����ѹǿԽ��A��ת����Խ�ߣ�˵���÷�Ӧ������Ӧ�����������С�ķ�Ӧ�����x=1����Ӧ����ʽΪ��
��100%=50%����ѹǿԽ��A��ת����Խ�ߣ�˵���÷�Ӧ������Ӧ�����������С�ķ�Ӧ�����x=1����Ӧ����ʽΪ��
A(g) + B(g) ![]() C(g)
C(g)
c(ʼ)(mol/L) 2 2 0
c(��)(mol/L) 1.22 1.22 1.22
c(ƽ)(mol/L) 0.78 0.78 1.22
���Ը÷�Ӧ��ƽ�ⳣ��K=![]() =2��
=2��
(4)�ٰ�ˮ�����ᷢ����ӦHCl+NH3H2O�TNH4Cl+H2O�����ݵ���غ㣺c(NH4+)+c(H+)=c(OH-)+c(Cl-)����Ӧƽ��ʱ��Һc(NH4+)=c(Cl-)������Һ��c(H+)=c(OH-)��������Һ�����ԣ�
��ƽ��ʱ����c(NH4+)=c(Cl-)����c(NH4+)=![]() =0.005mol/L�����������غ�ɵ�c(NH3H2O)=
=0.005mol/L�����������غ�ɵ�c(NH3H2O)=![]() mol/L-0.005mol/L=(
mol/L-0.005mol/L=(![]() -0.005)mol/L����ҺΪ���ԣ���c(OH-)=10-7mol/L������NH3H2O�ĵ��볣��Kb=
-0.005)mol/L����ҺΪ���ԣ���c(OH-)=10-7mol/L������NH3H2O�ĵ��볣��Kb=![]() ��
��

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�����Ŀ���������أ�![]() ���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
I.�Ʊ�
����1���Ʊ������ᶡ����![]() ��
��
![]()
��Ӧװ����ͼ1���г�װ����ȥ�������ձ������μ���ϡ���ᡢ����������������Һ������Ӧ��ȫ������ϲ���״���![]() ��
��![]() �Ļ����Һϴ�����Σ���������á�
�Ļ����Һϴ�����Σ���������á�
����2���Ʊ���������
![]()
��Ӧװ����ͼ2���гּ�����װ��·ȥ����������A�м���![]() �Ҵ���Һ��
�Ҵ���Һ��![]() ��������
��������![]() ���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������
���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������![]() ���
���


��������������£�
���� | ��ɫ��״̬ | �е㣨�棩 | �ܽ��� |
| ��ɫ���� | �����ֽ� | ������ˮ�������Ҵ������������� |
| ��ɫҺ�� | 118 | ����ˮ�����Ҵ������ѻ��� |
| ��ɫ��ɫ��״Һ�� | 78 | ������ˮ�����Ҵ������ѻ��� |
| ��ɫ��״Һ�� | 118 | ��ˮ���Ҵ����ܣ����������� |
��ش�
��1������A������Ϊ_____________.
��2������1�з���������ᶡ���IJ�������Ϊ_____________������1����NaCl��NaHCO3�Ļ����Һϴ�ӵ�Ŀ����__________________________.
��3������2�б�ԡ��ȴ��Ŀ����__________________________������2�и����Ʒ���¶ȿ�����55~60�棬ԭ����__________________________
��4��������߲�Ʒ�Ĵ��ȣ�����_____________�����ţ��н����ؽᾧ��
A.��ˮ�Ҵ� B.��ˮ���� C.ˮ D.�Ҵ���ˮ��Һ
��.�ֹ��ȷ��ⶨ��Ʒ�Ĵ���
ԭ����![]() ��
��![]() ��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�
��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�![]() ����ȡ�����ȷ����Ʒ��Һ�е�
����ȡ�����ȷ����Ʒ��Һ�е�![]() ���ⶨ�������£�
���ⶨ�������£�
����![]() Ʒ������
Ʒ������![]() ����Һ��
����Һ��
������һ����ͬ�����![]() ����ͬŨ�ȵ�
����ͬŨ�ȵ�![]() ����Һ���ֱ����
����Һ���ֱ����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��
����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��![]() ������ȵĹ�ϵ���ߣ���ͼ��
������ȵĹ�ϵ���ߣ���ͼ��
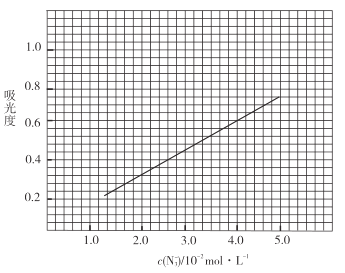
�۲�Ʒ�ⶨ����ȡ0.360g��Ʒ�����![]() ��Һ��ȡ��
��Һ��ȡ��![]() �ڱ����У�����
�ڱ����У�����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������Ϊ0.6��
����Һ��ҡ�ȣ���������Ϊ0.6��
��5��ʵ������![]() ��������
��������![]() ����Һ�ķ���Ϊ_________________.
����Һ�ķ���Ϊ_________________.
��6����Ʒ�Ĵ���Ϊ_________________�������м����![]() ����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����