��Ŀ����
����Ŀ����ҵ�����Ȼ�隣������̿��Ʊ��ߴ�̼���̵�������ͼ��ʾ��

��֪�������̿����Ҫ�ɷ���MnCO3�����к�Fe��Ca��Mg��Al��Ԫ�ء�
��Al3����Fe3��������ȫ��pH�ֱ�Ϊ4.7��3.2��Mn2����Mg2����ʼ������pH�ֱ�Ϊ8.1��9.1��
�۱��չ�������Ҫ��ӦΪMnCO3��2NH4Cl ![]() MnCl2��2NH3����CO2����H2O��
MnCl2��2NH3����CO2����H2O��
(1)���ͼ1��2��3���������չ�������ѵı����¶ȡ�����ʱ�䡢m(NH4Cl)/m(���̿��)�ֱ�Ϊ____________��____________��____________��

(2)�Խ���Һ��������ʱ�����ȼ���MnO2��Fe2��ת��ΪFe3�����ٵ�����ҺpH�ķ�Χ__����Fe3����Al3����Ϊ��������ȥ��Ȼ�����NH4F��Ca2����Mg2����Ϊ�����������ȥ��
(3)��̼���ᾧ�������У�����̼�����ʱ��Ӧ�����ӷ���ʽΪ____��
(4)���������п�ѭ��ʹ�õ�������________��
(5)���õζ����ⶨ����Һ��Mn2���ĺ�����ʵ�鲽�裺��ȡ1.000 g�����������м����Թ�������������ᣬ����ʹ��Ӧ2Mn2����NO3-��4PO43-��2H��![]() 2[Mn(PO4)2]3����NO2-��H2O��ֽ��в���ȥ�������������Թ���������泥�������ӦNO2-��NH4+===N2����2H2O�Գ�ȥNO2-������ϡ�����ữ����2.00 mol��L��110.00 mL��������隣���Һ���еζ��������ķ�ӦΪ[Mn(PO4)2]3����Fe2��===Mn2����Fe3����2PO43-����0.10 mol��L��110.00 mL����K2Cr2O7��Һǡ�ó�ȥ������Fe2����
2[Mn(PO4)2]3����NO2-��H2O��ֽ��в���ȥ�������������Թ���������泥�������ӦNO2-��NH4+===N2����2H2O�Գ�ȥNO2-������ϡ�����ữ����2.00 mol��L��110.00 mL��������隣���Һ���еζ��������ķ�ӦΪ[Mn(PO4)2]3����Fe2��===Mn2����Fe3����2PO43-����0.10 mol��L��110.00 mL����K2Cr2O7��Һǡ�ó�ȥ������Fe2����
������K2Cr2O7��Һ��Fe2����Ӧ(��ԭ������Cr3��)�����ӷ���ʽΪ___________��
���������̵���������Ϊ________��
���𰸡�500 �� 60 min 1.10 4.7��pH<8.1 Mn2����2HCO3-===MnCO3����CO2����H2O NH4Cl 6Fe2����Cr2O72-��14H��===6Fe3����2Cr3����7H2O 77%
��������
(1)����ͼ������������¶���500 �����ϡ�����ʱ����60 min���ϡ�![]() ��1.1���ϣ��̽����ʳ���90% ��
��1.1���ϣ��̽����ʳ���90% ��
(2)�������ӵ�Ŀ���ǽ�Fe3����Al3����Ϊ������ȥ�����ұ�֤Mn2�����ܳ�����
(3)��̼���ᾧ�������У�̼������������ӷ�Ӧ����̼���̳����Ͷ�����̼���壻
(4)��������ͼ������ѭ��ʹ�õ����ʣ�
(5) �ٸ��ݵ�ʧ�����غ���д����K2Cr2O7��Һ��Fe2����Ӧ�����ӷ���ʽ��
�ڸ��ݹ�ϵʽMn2����[Mn(PO4)2]3����Fe2�������̵�����������
(1)����ͼ������������¶���500 �����ϡ�����ʱ����60 min���ϡ�![]() ��1.1���ϣ��̽����ʳ���90% �����Ա��չ�������ѵı����¶ȡ�����ʱ�䡢
��1.1���ϣ��̽����ʳ���90% �����Ա��չ�������ѵı����¶ȡ�����ʱ�䡢![]() �ֱ�Ϊ500 �桢60 min�� 1.10��
�ֱ�Ϊ500 �桢60 min�� 1.10��
(2)�������ӵ�Ŀ���ǽ�Fe3����Al3����Ϊ������ȥ�����ұ�֤Mn2�����ܳ��������Ե�����ҺpH�ķ�ΧΪ4.7��pH<8.1��
(3)��̼���ᾧ�������У�̼������������ӷ�Ӧ����̼���̳����Ͷ�����̼���壬��Ӧ���ӷ���ʽ��Mn2����2HCO3-===MnCO3����CO2����H2O��
(4)��������ͼ����ѭ��ʹ�õ�������NH4Cl��
(5) �ٸ��ݵ�ʧ�����غ㣬����K2Cr2O7��Һ��Fe2����Ӧ�����ӷ���ʽ��6Fe2����Cr2O72-��14H��===6Fe3����2Cr3����7H2O��
��Cr2O72-�����ʵ�����0.10 mol��L��1��0.01L=0.001mol����Ӧ6Fe2����Cr2O72-��14H��===6Fe3����2Cr3����7H2O����Fe2�������ʵ�����0.006mol�� [Mn(PO4)2]3����Fe2��===Mn2����Fe3����2PO43-����Fe2�������ʵ�����2.00 mol��L��1��0.01L��0.006mol=0.014mol������Mn2����[Mn(PO4)2]3����Fe2������֪Mn2�������ʵ�����0.014mol���̵���������Ϊ![]() ��
��

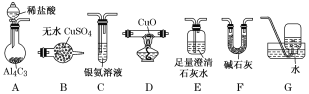
����Ŀ�������ڼ��������¿ɻ�ԭ����ͭ����������ˮ�����⣬����̼�������ij��ѧС��������ͼװ��̽���䷴Ӧ���
[��������]��CO����������Һ��Ӧ��CO��2[Ag(NH3)2]����2OH��===2Ag����2NH4+��CO32����2NH3��
��Cu2OΪ��ɫ������Ag+��Ӧ���ܷ�����Ӧ��Cu2O��2H��===Cu2+��Cu��H2O��
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�������������װ�ô����ҵ�����˳��ΪA��__________________��(����ĸ���)
��3��ʵ���еμ�ϡ����IJ���Ϊ______________________________________________��
��4����֪��������к���CO����װ��C�пɹ۲쵽��������________________��װ��F������Ϊ_________________________________________��
��5������Ӧ������װ��D���Թ��й���ȫ����Ϊ��ɫ��
�����ʵ��֤����ɫ�����к���Cu2O��______________________________________________��
����֤����ɫ�������Ƿ���Cu����ͬѧ�������ʵ�飺��������ɫ�����м�������0.1mol��L1AgNO3��Һ��������Һ�������ݴ��жϺ�ɫ�����к���Cu����ͬѧ��Ϊ�÷�������������֤����ͬѧ�Ľ��ۣ������������¶Ա�ʵ�飬��ɱ������ݡ�
ʵ�鲽��(��Ҫ��д�������������) | Ԥ������ͽ��� |
__________________ | ���۲쵽��Һ����������֤����ɫ�����к���Cu�����۲쵽��Һ����������֤����ɫ�����к���Cu |