��Ŀ����
����Ŀ��I��һ���¶��£�ij�ݻ�Ϊ2 L���ܱ������ڣ�ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯����������ͼ����ͼ��ʾ��
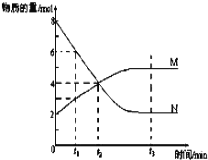
��1���÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
��2����ͼ����ʾ������ʱ���У�_______����t1��t2��t3��ʱ�̴ﵽ��ѧ��Ӧ����
II��һ���¶��½�6 mol A��6 mol B�����2 L���ܱ������У�
�������·�Ӧ��3A(g)+B(g)![]() xC(g)+2D(g)������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60%��C��ƽ����Ӧ������0.36 mol/��L��min������
xC(g)+2D(g)������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60%��C��ƽ����Ӧ������0.36 mol/��L��min������
��1��ƽ��ʱD��Ũ��=___________________��
��2��B��ƽ����Ӧ������(B)= ___________________________��
��3��x=_________��
��4����ʼʱ�����е�ѹǿ��ƽ��ʱ��ѹǿ֮��Ϊ______________����Ϊ��������ȣ���
���𰸡� 2N![]() M t3 1.2 mol��L�C1 0.12 mol��L�C1��min�C1 3 10:11
M t3 1.2 mol��L�C1 0.12 mol��L�C1��min�C1 3 10:11
��������(1)��ͼ��֪��N����8-2=6��M����5-2=3��NΪ��Ӧ�MΪ������ұ仯��֮��Ϊ2��1������ѧ������֮��Ϊ2��1����ӦΪ2N(g) ![]() M(g)���ʴ�Ϊ��2N(g)
M(g)���ʴ�Ϊ��2N(g) ![]() M(g)��
M(g)��
(2)�����ʵ�����ʱ��ı仯���������仯ʱΪƽ��״̬����ͼ��֪��t3Ϊƽ��״̬���ʴ�Ϊ��t3��
3A(g)+B(g) ![]() xC(g)+2D(g)������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60%����
xC(g)+2D(g)������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60%����
3A(g)+B(g) ![]() xC(g)+2D(g)��
xC(g)+2D(g)��
��ʼ(mol/L) 33 00
ת��3��60%0.60.6x1.2
ƽ��1.22.40.6x1.2
(1)������������֪ƽ��״̬D��Ũ��Ϊ1.2mol/L���ʴ�Ϊ��1.2mol/L��
(2)B��ƽ����Ӧ���ʦ�(B)= ![]() =0.12mol/(L��min)���ʴ�Ϊ��0.12mol/(L��min)��
=0.12mol/(L��min)���ʴ�Ϊ��0.12mol/(L��min)��
(3)������֮�ȵ��ڻ�ѧ������֮�ȿ�֪�� ![]() =
=![]() �����x=3���ʴ�Ϊ��3��
�����x=3���ʴ�Ϊ��3��
(4)��ʼʱ�����е�ѹǿ��ƽ��ʱ��ѹǿ֮�ȵ��ڷ�Ӧǰ������ʵ���֮�͵ıȣ�Ϊ(6+6)mol��(1.2+2.4+0.6��3+1.2)mol/L��2L=10��11���ʴ�Ϊ��10��11��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
