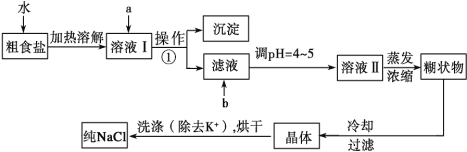
��Ŀ����
����Ŀ��I����һ���¶��£���a.���� b.���� c.���������
������c(H��)��ͬ�����Ҳ��ͬʱ���ֱ����������п����ͬ״���²������������ɴ�С��˳����_________��������ű�ʾ����ͬ��
II�������£���Ũ�Ⱦ�Ϊ1 mol��L��1������������Һ��
��H2SO4��Һ ��NaHCO3��Һ ��NH4Cl��Һ ��NaOH��Һ
��1����ҺpH�ɴ�С��˳���� ��������ˮ�����H��Ũ����С���� ����������ţ�
��2�����и�����Ũ���ɴ�С��˳����_____________ �������¶ȣ�NH4Cl��Һ��pH______����������������������������������
��3��������ͨ��������������ʱ![]() ��ֵ_________(����������������С������������)��
��ֵ_________(����������������С������������)��
��4������������Ϻ���Һǡ�ó����ԣ�����ǰ������� �������(��������������С��������������)��
���𰸡�I.c��a=b��3�֣���.(1)����������������3�֣�������2�֣�
��2��c(Cl��)��c(NH4+)��c(H+)��c(OH��)��3�֣������ͣ�2�֣�
��3����С��2�֣���4�����ڣ�2�֣�
��������
��������� I.�����������ǿ�ᣬ��ȫ���룬���Ե�������Ũ�Ⱥ������ͬʱ�������ṩ�����������ʵ�����ȣ�����������������ȡ������������ᣬ���ֵ��룬���������������Ũ����������ͬʱ�������Ũ�ȱ���������ṩ�����������ʵ���������࣬��������������࣬���������������С˳��Ϊc��a=b��
��.��1�������Ƕ�Ԫǿ�ᣬpH��7��̼������ˮ���Լ��ԣ��Ȼ��ˮ�������ԣ�����������һԪǿ��Լ��ԣ�����ˮ��̶ȶ���С���������Ի���Զ������������Һ��pH��˳��Ϊ���������������������������������ˮ�ĵ��룬��������������������Ũ�ȴ����������Ƶ����������������Ũ�ȣ���ˮ�ĵ����������ø�ǿ��������������ˮ�ĵ���̶���С��ѡ����
��2���Ȼ����ȫ����������Ӻ�笠����ӣ���Ϊ笠�����ˮ�������ԣ���������Ũ��˳��Ϊc(Cl��)��c(NH4+)��c(H+)��c(OH��)�����£��ٽ�笠�����ˮ�⣬��Һ��pH���͡�
��3���Ȼ��������ͨ�백��������笠�����ˮ�⣬����笠�����Ũ�ȼ�С��һˮ�ϰ�Ũ������ֵ��С����4���Ȼ�狀��������Ƶ������Ϻ������Ȼ��ƺ�һˮ�ϰ�����Һ�Լ��ԣ�Ҫ�����ԣ����Ȼ�粒��������Ȼ�淋���������������Ƶ������

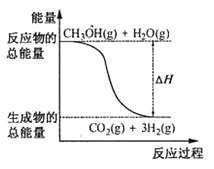
����Ŀ���������ȣ�ClO2���Ǽ�������ˮ�Ҳ���ˮ������ѧ��Ӧ�Ļ���ɫ���壬�е�Ϊ11�棬�����ڴ��������ˮ��ijС����ʵ������̽��ClO2��Na2S�ķ�Ӧ���ش��������⣺
(l)ClO2���Ʊ�������֪��SO2+2NaClO3+H2SO4=2ClO2��+2NaHSO4��
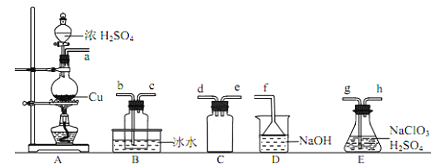
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_______________��
�����ռ������ClO2��ѡ����ͼ�е�װ�ã�������˳��Ϊ a��_________(������������Сд��ĸ��ʾ)
��װ��D��������_________��
��2��ClO2�� Na2S �ķ�Ӧ
�������ռ�����ClO2�� N2ϡ������ǿ���ȶ��ԣ�����������ϡ�ͺ��ClO2ͨ����ͼ��ʾװ���г�ַ�Ӧ���õ���ɫ������Һ��һ��ʱ���ͨ������ʵ��̽�� I �з�Ӧ�IJ��
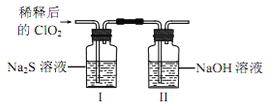
�������� | ʵ������ | ���� |
ȡ����������Һ���Թܼ��У� ����Ba(OH)2��Һ���� | ��_________ | ��������������� |
��ȡ����������Һ���Թ����У� �μ�Ʒ����Һ������ | Ʒ����Һ����ɫ | �� ��_____���� |
�����Թܼ��м�����Ba(OH)2��Һ�� ���������ã�ȡ�ϲ���Һ���Թܱ���______ | �а�ɫ�������� | ��Cl-���� |
��ClO2��Na2S��Ӧ�����ӷ���ʽΪ__________�����ڴ��������ˮʱ��ClO2�����Cl2���ŵ���____________(��д2��)��