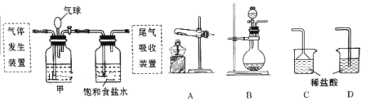
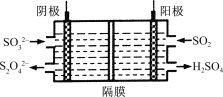
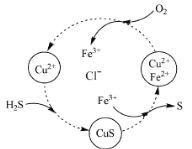
��Ŀ����
����Ŀ�������£����и������ӻ������ָ����Һ�п��ܴ����������
A��pH��7����Һ��Fe3+��NH4+��Cl-��NO3-
B��ˮ�������c��H+����1.0��10-12mol��L-1����Һ�У�Na+��SO42-��HCO3-��K+
C����ˮ�������c��OH-����1.0��10-2mol��L-1����Һ��Cl-��CH3COO-��K+��[Al��OH��4]-
D��![]() ��1012����Һ�У�NH4+��Al3+��NO3-��Cl-
��1012����Һ�У�NH4+��Al3+��NO3-��Cl-
���𰸡�C
��������
���������A��Fe3+����ˮ�⣬��Һ�����ԣ���pH��7����Һ�������ڣ���A����B��ˮ�������c��H+����1.0��10-12mol��L-1����Һ���������ԣ�Ҳ�����Լ��ԣ�HCO3-���������Һ������ܴ������ڣ���B����
C����ˮ�������c��OH-����1.0��10-2mol��L-1����Һ���������ԣ�Ҳ�����Լ��ԣ�Cl-��CH3COO-��K+��[Al��OH��4]-�ڼ��������¾��ܴ������ڣ���C��ȷ��D��![]() ��1012����Һ�Լ�����NH4+��Al3+�������ڼ�����Һ�д������ڣ���D����ΪC��
��1012����Һ�Լ�����NH4+��Al3+�������ڼ�����Һ�д������ڣ���D����ΪC��

��ϰ��ϵ�д�
�����Ŀ