��Ŀ����
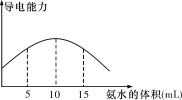
����Ŀ����1��ȡ20 mL pH��3��CH3COOH��Һ������0.2 mol��L��1�İ�ˮ�������Һ�����Ա仯��ͼ������백ˮǰCH3COOH�ĵ����(���ӵ���İٷ���)Ϊ______________������0��10 mL�İ�ˮ����������ǿ��ԭ��_________________________��
��2��������粒�������ˮ�����0.1 mol��L��1��Һ����֪����ĵ���ƽ�ⳣ��ΪKa��һˮ�ϰ��ĵ���ƽ�ⳣ��ΪKb��ʵ�鷢�����߽�����ȣ���д�������ˮ������ӷ���ʽ______________________��ˮ��ƽ�ⳣ���ı���ʽ____________________��
���𰸡�1% �����кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ NH4+��CH3COO����H2O![]() NH3��H2O��CH3COOH K��
NH3��H2O��CH3COOH K��![]()
��������
(1)����ͼ�����������ˮ�����Ϊ10mLʱ��������ǿ��˵���պ��������ȫ��Ӧ�����Դ����Ũ��Ϊ��![]() =0.1molL-1���ٸ���pH=3��CH3COOH��Һ����������Ũ��Ϊ10-3molL-1������CH3COOH�ĵ����Ϊ
=0.1molL-1���ٸ���pH=3��CH3COOH��Һ����������Ũ��Ϊ10-3molL-1������CH3COOH�ĵ����Ϊ ![]() ��100%=1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ����˼���0��10 mL�İ�ˮ������������ǿ���ʴ�Ϊ��1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ��
��100%=1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ����˼���0��10 mL�İ�ˮ������������ǿ���ʴ�Ϊ��1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ��
(2)����������������Σ�������ǿ����ʣ�����ˮ����ȫ���룬笠����Ӻ���������Ӷ�ˮ�⣬��ѧ����ʽΪCH3COONH4+H2OCH3COOH+NH3H2O�����ӷ���ʽΪ��CH3COO-+NH4++H2OCH3COOH+NH3H2O��ˮ��ƽ�ⳣ���ı���ʽK=![]() =
=![]() =
=![]() =
=![]() ���ʴ�Ϊ�� CH3COO-+NH4++H2OCH3COOH+NH3H2O��K=
���ʴ�Ϊ�� CH3COO-+NH4++H2OCH3COOH+NH3H2O��K=![]() ��
��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�