��Ŀ����
����Ŀ���⻯ﮣ�LiH���ڸ���Ŀ��������ȶ����ڣ���ˮ�����ܹ�����ȼ�ա�ij�С����ʹ������װ���Ʊ�LiH���塣
��ͬѧ��ʵ�鷽�����£�
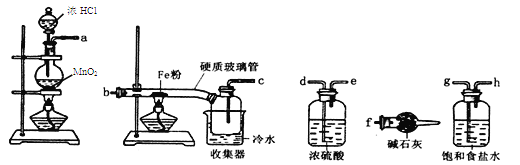
��1����������װ���ӣ���������װ�ð���������������˳��Ϊ________________������ҩƷǰ����Ҫ���е�ʵ�������____________������д������IJ���������������װ��B��������___________��
��2������ҩƷ�������Ӵ��Լ�ƿ��ȡ��һ��������ﮣ�����ʯ���ܷ⣩��Ȼ���ڼױ��н�ϴ���Σ��ò�����Ŀ����____________________��Ȼ����ٰ�﮷��뵽ʯӢ���С�
��3��ͨ��һ��ʱ�����������ʯӢ�ܣ�ͨ������������___________________________���ڼ���D����ʯӢ��֮ǰ��������е�ʵ�������__________��
��4������һ��ʱ���ֹͣ���ȣ�����ͨ������ȴ��Ȼ��ȡ��LiH��װ�뵪���ƿ������ڰ�������ȡ����������Ŀ����Ϊ�˱���LiH������е�ˮ�����Ӵ�������Σ�գ���Ӧ����ʽΪ_____________��
��5��ȷ�����ƵõIJ�Ʒ0.174g����һ��������������ˮ��Ӧ���ռ�������470.4 mL���ѻ���ɱ�״���������Ʒ��LiH��Li�����ʵ���֮��Ϊ____________________��
���𰸡� e��a��b��f��g��d��c ��f��g����Ҳ���ԣ� ����װ�õ������� ��ȥH2�е�H2O��HCl ��ȥﮱ����ʯ�� ����װ���п�������ֹ����ʱﮡ�������������Ӧ��������ը �ռ�c���ų������岢����H2���� LiH + H2O = LiOH + H2�� 10��1
���������������ɸ�װ�õ��ص�����ƶ�����ͬѧ��ʵ�鷽��Ϊ���£���Aװ����п�������Ʊ�����������������һ������H2O��HCl��������B�ü�ʯ�ҳ�ȥH2�е�H2O��HCl��ͨ��D���ų�ϵͳ�ڵĿ������������Ĵ��ȣ�Ȼ�����D�в������Ʊ�LiH��Cװ����Ϊ�˷�ֹ�����е�ˮ��������D������ֹ�⻯ﮣ�LiH����ˮ����ȼ�ա�
��⣺��1����������װ���ӣ���������װ�ð���������������˳��Ϊe��a��b��f��g��d��c ��f��g����Ҳ���ԣ�������ҩƷǰ����Ҫ���е�ʵ������Ǽ���װ�õ������ԣ�����װ��B�������dz�ȥH2�е�H2O��HCl��
��2�������Ӵ��Լ�ƿ��ȡ��һ��������ﮣ�����ʯ���ܷ⣩��Ȼ���ڼױ��н�ϴ���Σ�������������ԭ����֪��ʯ�������ڱ����ʸò�����Ŀ���dz�ȥﮱ����ʯ����
��3��ͨ��һ��ʱ�����������ʯӢ�ܣ�ͨ�����������Ǹ���װ���п�������ֹ����ʱﮡ�������������Ӧ��������ը���ڼ���D����ʯӢ��֮ǰ��������е�ʵ��������ռ�c���ų������岢����H2������
��4������һ��ʱ���ֹͣ���ȣ�����ͨ������ȴ��Ȼ��ȡ��LiH��װ�뵪���ƿ������ڰ�������ȡ����������Ŀ����Ϊ�˱���LiH������е�ˮ�����Ӵ�������Σ�գ���Ӧ����ʽΪLiH + H2O = LiOH + H2����
��5��ȷ�����ƵõIJ�Ʒ0.174g����һ��������������ˮ��Ӧ���ռ�������470.4 mL���ѻ���ɱ�״��������n(H2)=![]() ����n(LiH)
����n(LiH)![]() ��n(H2)=n(LiH)+0.5
��n(H2)=n(LiH)+0.5![]() ����֮��n(LiH)=0.02mol��
����֮��n(LiH)=0.02mol��![]() �����ԣ���Ʒ��LiH��Li�����ʵ���֮��Ϊ10��1��
�����ԣ���Ʒ��LiH��Li�����ʵ���֮��Ϊ10��1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ҵ���ú����۷���V2O3��Ϊ����ijʯúΪԭ�ϣ�����Al2O3��CaO�����ʣ����ƻ��������Ʊ�V2O5�����������£�
![]()
����������+5�۷�����Һ�е���Ҫ������ʽ����ҺpH�Ĺ�ϵ��
pH | 4~6 | 6~8 | 8~10 | 10~12 |
��Ҫ���� | VO2+ | VO3 | V2O74 | VO43 |
��1�����գ���ʯú�м���ʯ�ұ�������V2O3ת��ΪCa(VO3)2�Ļ�ѧ����ʽ��______��
��2������� �� Ca(VO3)2������ˮ�����������ᡣ����ɰ���ʱ��Һ��pH��4��Ca(VO3)2������������ӷ���ʽ��______��
�� ��ȶԷ��������ܽ�����Ӱ����ͼ��ʾ�����ʱ��Һ����ȿ����ڴ�Լ3.2%��������ͼ�Ʋ������ʱ��ѡ�������ȵ�ԭ����______��

��3��ת����������Һ�еķ�ת��ΪNH4VO3���壬���������£�
![]()
�� ����Һ�м���ʯ�����������______��
�� ��֪CaCO3���ܽ��С��Ca3(VO4)2����Ca3(VO4)2�����м���(NH4)2CO3��Һ����ʹ���ӳ������ܳ�����ϻ�ѧ�����ƽ���ƶ�ԭ��������ԭ����______��
�� ��(NH4)3VO4��Һ�м���NH4Cl��Һ��������Һ��pH��7.5����pH��8ʱ��NH4VO3�IJ������Խ��ͣ�ԭ����______��
��4���ⶨ��Ʒ��V2O5�Ĵ��ȣ�
��ȡa g��Ʒ�����������ܽ����õ�(VO2)2SO4��Һ���ټ���b1 mL c1 mol��L1 (NH4)2Fe(SO4)2��Һ��VO2+ + 2H+ + Fe2+ == VO2+ + Fe3+ + H2O���������c2 mol��L1 KMnO4��Һ�ζ�������(NH4)2Fe(SO4)2���յ㣬����KMnO4��Һ�����Ϊb2 mL����֪ MnO4����ԭΪMn2+���������ʲ����뷴Ӧ�����Ʒ��V2O5������������______����V2O5��Ħ��������182 g��mol1��