��Ŀ����
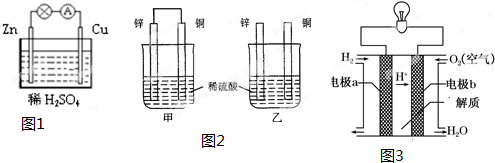
11����A��B��C��D�Ŀ����Ƭ����������ʵ�飬��A��B�õ���������ͬʱ����ϡH2SO4�У�A��Ϊ���� ��C��D�õ���������ͬʱ����ϡH2SO4�У�������C�����ߡ�D ��A��C������ͬʱ����ϡH2SO4��C�������������� ��B��D������ͬʱ����ϡH2SO4�У�D������������Ӧ�������ֽ����Ļ��˳��Ϊ��������| A�� | A��B��C��D | B�� | A��C��D��B | C�� | C��A��B��D | D�� | B��D��C��A |
���� һ����˵��ԭ����н�����Ը������������ϣ����ӴӸ����ص�������������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ���ݴ˷������
��� �⣺һ����˵��ԭ����н�����Ը������������ϣ�
A��B�õ���������ͬʱ����ϡH2SO4�У�A��Ϊ��������������A��B��
��C��D�õ���������ͬʱ����ϡH2SO4�У�������C�����ߡ�D����C�Ǹ�����D���������������C��D��
��A��C������ͬʱ����ϡH2SO4��C�������������ݣ���C�ϵõ��ӷ�����ԭ��Ӧ��C��������A�Ǹ������������A��C��
��B��D������ͬʱ����ϡH2SO4�У�D������������Ӧ����D�Ǹ�����B���������������D��B��
ͨ�����Ϸ���֪���������˳��A��C��D��B����ѡB��
���� ������ԭ���ԭ������������˳����ȷ�ж��������������������������Թ�ϵ�ǽⱾ��ؼ���֪��ԭ����������Ϸ�Ӧ���͡���Ӧ������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
10��������������ȷ���ǣ�������
| A�� | 1����ϩ������8���Ҽ���1���м� | |
| B�� | 1��4-���ױ��˴Ź���������������壬����ԭ����֮��Ϊ3��2 | |
| C�� | ����������������Է��� | |
| D�� | ����ʽΪC2H6O�ĺ������ͼ�Ϸ�����C-H����C-O���������գ��ɴ˿��Գ����Ʋ��л���ṹ��ʽΪC2H5-OH |
6�����о������ڷ��Ӿ�����ǣ�������
| A�� | ʯӢ | B�� | C60 | C�� | NaCl | D�� | ����K |
16��Ϊ������Һ���Ƿ���Cl-��ijͬѧ��������Һ���ȼ�HNO3���ټ�AgNO3����Һ��ʵ�鷽�������а�ɫ�������ɣ���֤����Cl-���Դ˽��ۣ�������������ɣ����������̽����ʵ�飮
ʵ��һ����Na2SO4��Һ�еμ�AgNO3��Һ
��1��ʵ��һ�в������������ӷ���ʽΪ2Ag++SO42-�TAg2SO4����
��2��ѧ����������±���ʵ��һ���ݽ������ۼ��㣬�������ɱ������в�Ҫ���ո�
[25��ʱKsp��Ag2SO4��=1.2��10-5��Ksp��AgCl��=1.8��10-10]
����l mLij��Һ�м���3��0.1mol/L AgNO3��Һ�������������ݣ��ж�����˵����ȷ����AD ������ĸ��ţ���
A�����Һ��c��SO42-��=0.1mol/Lʱ�������Ag2SO4����
B�����Һ��c��SO42-��=1mol/Lʱ�������Ag2SO4����
c������SO42-Ũ�ȴ�С�������Ag2SO4����
D����ʹ��0.01mol/L AgNO3��Һ���ɻ����ų�SO42-��Cl-���鹹�ɵĸ���
��3����ʵ��һ�б�Ţ��е����ۼ�������������գ����������ϴ�Ag+Ӧ���γɳ��������롰��Щ�����ǡ���������ì�ܣ�Ϊ̽�����࣬��ʵ��һ�Ļ����ϼ������������ʵ�飮
ʵ�����
����Ag2SO4���������ԭ����������¼��裬����ɼ���һ������֪��H2SO4=H++HSO4-��HSO4-=H++SO42-����������ܵ�ԭ����NO3-��Ag+�γ���λ�����
����һ��H+��Ag2SO4�ܽ������ã�
�������NO3-��Ag2SO4�ܽ������ã�
��4����������ѡ�Լ���ѡ���ʵ��Լ������ʵ�鷽�����ֱ���֤����һ�ͼ�����Ƿ�������� д��ʵ�鲽��ͽ��ۣ�����ѡ�Լ���Ag2SO4���塢ŨHNO3��NaNO3������Һ��CaSO4���壩��ȡ����CaSO4�������Թ��У�����һ����ŨHNO3��������������ܽ⣬˵������һ������
��ȡ����Ag2SO4�������Թ��У�����һ����NaNO3������Һ��������������ܽ⣬˵�����������
��5��ͨ����4����ʵ�飬��֤ʵ����һ����������ƽ�����۽���Ag2SO4�ܽ��ԭ��Ag2SO4������ˮ�д���ƽ�⣺Ag2SO4��s��?2Ag+��aq��+SO42-��aq����H+��SO42-�������HSO4-��SO42-Ũ�Ƚ��ͣ�ƽ�������ƶ���Ag2SO4�����ܽ⣮
ʵ��һ����Na2SO4��Һ�еμ�AgNO3��Һ
| ��� | Na2S04��Һ | AgN03��Һ | ���� | ||
| ���/mL | Ũ��/��mol•L-1�� | ���/�� | Ũ��/��mol•L-1�� | ||
| �� | 1 | l | 3 | 2 | ���ִ�����ɫ���� |
| �� | 1 | 1 | 3 | 0.5 | ����������ɫ���� |
| �� | 1 | 1 | 3 | 0.1 | �������� |
| �� | 1 | 1 | 3 | 0.0l | �����Ա仯 |
��2��ѧ����������±���ʵ��һ���ݽ������ۼ��㣬�������ɱ������в�Ҫ���ո�
[25��ʱKsp��Ag2SO4��=1.2��10-5��Ksp��AgCl��=1.8��10-10]
| ��� | AgNO3Ũ��/��mol•L-1�� | ϡ�ͺ�Ag+Ũ��/��mol•L-1�� | ���Һ��SO42-����С���ۼ��Ũ��/��mol•L-1�� |
| �� | 2 | 0.2 | 0.0003 |
| �� | 0.5 | 0.0048 | |
| �� | 0.1 | 0.0l | 0.12 |
| �� | 0.001 |
A�����Һ��c��SO42-��=0.1mol/Lʱ�������Ag2SO4����
B�����Һ��c��SO42-��=1mol/Lʱ�������Ag2SO4����
c������SO42-Ũ�ȴ�С�������Ag2SO4����
D����ʹ��0.01mol/L AgNO3��Һ���ɻ����ų�SO42-��Cl-���鹹�ɵĸ���
��3����ʵ��һ�б�Ţ��е����ۼ�������������գ����������ϴ�Ag+Ӧ���γɳ��������롰��Щ�����ǡ���������ì�ܣ�Ϊ̽�����࣬��ʵ��һ�Ļ����ϼ������������ʵ�飮
ʵ�����
| ��� | AgNO3��Һ Ũ��/��mol•L-1�� | ���� | ������еμ����������� |
| �� | 2 | ���ִ�����ɫ���� | �μ�ϡ���ᣬ���������ܽ⣻�ļ�Ũ���ᣬ�����Ͽ���ʧ |
| �� | 0.5 | ����������ɫ���� | �μ�ϡ���ᣬ����������ʧ |
����һ��H+��Ag2SO4�ܽ������ã�
�������NO3-��Ag2SO4�ܽ������ã�
��4����������ѡ�Լ���ѡ���ʵ��Լ������ʵ�鷽�����ֱ���֤����һ�ͼ�����Ƿ�������� д��ʵ�鲽��ͽ��ۣ�����ѡ�Լ���Ag2SO4���塢ŨHNO3��NaNO3������Һ��CaSO4���壩��ȡ����CaSO4�������Թ��У�����һ����ŨHNO3��������������ܽ⣬˵������һ������
��ȡ����Ag2SO4�������Թ��У�����һ����NaNO3������Һ��������������ܽ⣬˵�����������
��5��ͨ����4����ʵ�飬��֤ʵ����һ����������ƽ�����۽���Ag2SO4�ܽ��ԭ��Ag2SO4������ˮ�д���ƽ�⣺Ag2SO4��s��?2Ag+��aq��+SO42-��aq����H+��SO42-�������HSO4-��SO42-Ũ�Ƚ��ͣ�ƽ�������ƶ���Ag2SO4�����ܽ⣮
1�����й�ϵʽ�����ӷ���ʽ�У���ȷ���ǣ�������
| A�� | ��NH4Al��SO4��2��Һ�е���Ba��OH��2��Һǡ��ʹSO42-��ȫ������NH4++Al3++2SO42-+2Ba2++5OH-=AlO2-+NH3•H2O+2BaSO4�� | |
| B�� | �����£�0.1 mol•L-1Na2S��Һ�д��ڣ�c��OH-��=c��H+��+c��HS-��+c��H2S�� | |
| C�� | �����£���0.1 mol•L-1CH3COOH��Һ��ˮϡ�ͣ�����Һ��pH��3.0����4.0ʱ����Һ��$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$��ֵ����ԭ����10�� | |
| D�� | �����£�0.1 mol•L-1HA��Һ��0.1 mol•L-1NaOH��Һǡ����ȫ��Ӧʱ����Һ��һ�����ڣ�c��Na+��=c��A-����c��OH-��=c��H+�� |
 H++H2PO4-��H2PO4-
H++H2PO4-��H2PO4- 
 ��
��
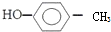
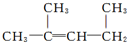 �����ƣ�1��3-����-2��ϩ��������
�����ƣ�1��3-����-2��ϩ��������