��Ŀ����
����Ŀ���������糧�ͷų���������������(NOx)��SO2��CO2���������ɻ������⡣��ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ġ�
(1)���������ü������ԭNOx��
CH4(g)�� 4NO2(g)��4NO(g)�� CO2(g)�� 2H2O(g) ��H1����574 kJ/mol
CH4(g)�� 4NO(g)��2N2(g)�� CO2(g)�� 2H2O(g) ��H2����1160 kJ/mol
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ__________________________��
(2)��̼����CO2ת��Ϊ�״���CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)��H3
CH3OH(g)��H2O(g)��H3
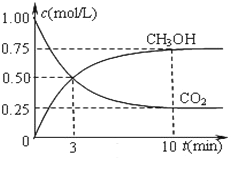
��һ���º����ܱ������г���1 mol CO2��3 mol H2���з�Ӧ�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ���ش�0��10 min�ڣ�������ƽ����Ӧ����Ϊ___mol/(L��S)����10 min�����¶Ȳ��䣬����ܱ��������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��_________(����������������������������)�ƶ���
������ͼ��ʾC��D��E��F��X��Y���Ƕ��Ե缫������Դ��ͨ����(��)�е����̪��Һ����F�������Ժ�ɫ��
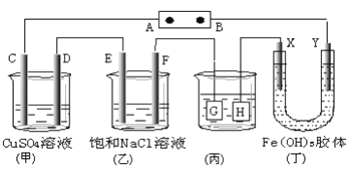
(1)���ü״�������ȼ�ϵ������Դ�������ΪKOH��Һ����A���ĵ缫��ӦʽΪ_________________________
(2)����(��)װ�ø�ͭ��������Ӧ���� _____�缫(��G��H)
(3)(��)װ����Y���������ɫ��_______(�����dz)
(4)ͨ��һ��ʱ���C��D��E��F�缫���е������ɣ������ʵ���֮��Ϊ_____________
���𰸡�CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g)��H��-867 kJ/mol 0.00375 ���� O2 + 4e- + 2H2O = 4OH- G �� 1:2:2:2
��������
���ݻ�ѧƽ�������ʽ���м�������ʵ�Ũ�Ⱥ�ƽ�ⳣ��������Ũ���̺�ƽ�ⳣ���Ĺ�ϵ������Ӧ���еķ����ݵ��ԭ�������������еĵ缫��Ӧ������
(1)���ݸ�˹���ɷ������� CH4(g)�� 4NO2(g)��4NO(g)�� CO2(g)�� 2H2O(g) ��H1����574 kJ/mol����CH4(g)�� 4NO(g)��2N2(g)�� CO2(g)�� 2H2O(g) ��H2����1160 kJ/mol
��+�ڿɵ��Ȼ�ѧ����ʽΪCH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g)��H����1160��574 =-867 kJ/mol��
(2).���ݷ���ʽ����
CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)
CH3OH(g)��H2O(g)
��ʼŨ��1.0 3.0 0 0
�ı�Ũ�� 0.75 2.25 0.75 0.75
ƽ��Ũ��0.25 0.75 0.75 0.75
0��10 min�ڣ�������ƽ����Ӧ����Ϊ2.25mol/L��600s=0.00375mol/(L��s)��ƽ�ⳣ��Ϊ![]() ����10 min�����¶Ȳ��䣬����ܱ��������ٳ���1 mol CO2(g)��1 mol H2O(g)����Qc=
����10 min�����¶Ȳ��䣬����ܱ��������ٳ���1 mol CO2(g)��1 mol H2O(g)����Qc=![]() <K��ƽ��������С�
<K��ƽ��������С�
����(��)�е����̪��Һ����F�������Ժ�ɫ��˵��F��Ϊ�����ӷ�Ӧ������������Һ��ʣ�����������ӣ�����FΪ��������Ӧ��BΪ������AΪ������ (1) ���ü״�������ȼ�ϵ������Դ�������ΪKOH��Һ��A Ϊ�������缫��ӦΪO2 + 4e- + 2H2O = 4OH- ��
(2) ����(��)װ�ø�ͭ��������Ӧ������������ΪG����
(3)��Ϊ������������ĵ�Ӿ����Ϊ��������������������ɣ�����������Y�������ɫ���
(4)ͨ��һ��ʱ���C������������D�ϲ���ͭ��E�ϲ���������F�ϲ������������ݵ����غ�������缫�ϵIJ�������ʵ�����Ϊ1:2:2:2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�








