题目内容
【题目】草酸亚铁晶体(FeC2O4·2H2O)是一种黄色难溶于水的固体,受热易分解,是生产电池、涂料以及感光材料的原材料。为探究纯净草酸亚铁晶体热分解的产物,设计装置图如下:
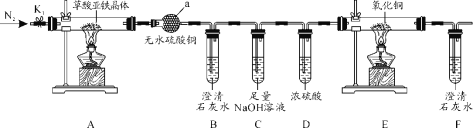
(1)仪器a的名称是______。
(2)从绿色化学考虑,该套装置存在的明显缺陷是_________。
(3)实验前先通入一段时间N2,其目的为__________。
(4)实验证明了气体产物中含有CO,依据的实验现象为_______。
(5)草酸亚铁晶体在空气易被氧化,检验草酸亚铁晶体是否氧化变质的实验操作是____。
(6)称取5.40g草酸亚铁晶体用热重法对其进行热分解,得到剩余固体质量随温度变化的曲线如下图所示:
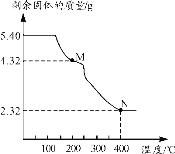
①上图中M点对应物质的化学式为_________。
②已知400℃时,剩余固体是铁的一种氧化物,试通过计算写出M→N发生反应的化学方程式:_______。
【答案】球形干燥管(干燥管) 缺少处理CO尾气装置 排尽装置内空气,防止空气中H2O和CO2的干扰 E中黑色粉末变红色,F出现白色沉淀 取少量草酸亚铁晶体于试管中,加入稀硫酸溶解后并滴加KSCN溶液,若溶液变红色,则草酸亚铁晶体已氧化变质;若不变红色,则草酸亚铁晶体未氧化变质 FeC2O4 3FeC2O4 ![]() Fe3O4+2CO2↑+4CO↑
Fe3O4+2CO2↑+4CO↑
【解析】
装置A为草酸亚铁晶体分解,利用无水硫酸铜检验水蒸气,B装置检验二氧化碳,C装置吸收二氧化碳,D装置干燥气体,E装置检验CO,F装置检验二氧化碳,据此解答。
(1)仪器a的名称是球形干燥管(干燥管),故答案为:球形干燥管(干燥管);
(2)反应会产生CO,缺少处理CO尾气装置,故答案为:缺少处理CO尾气装置;
(3)反应会会产生CO和H2O,通入氮气,排尽装置内空气,防止空气中H2O和CO2的干扰,故答案为:排尽装置内空气,防止空气中H2O和CO2的干扰;
(4)CO与CuO反应,生成Cu和二氧化碳,现象为E中黑色粉末变红色,F出现白色沉淀,故答案为:E中黑色粉末变红色,F出现白色沉淀;
(5)检验铁离子的试剂为KSCN,具体有:取少量草酸亚铁晶体于试管中,加入稀硫酸溶解后并滴加KSCN溶液,若溶液变红色,则草酸亚铁晶体已氧化变质;若不变红色,则草酸亚铁晶体未氧化变质,故答案为:取少量草酸亚铁晶体于试管中,加入稀硫酸溶解后并滴加KSCN溶液,若溶液变红色,则草酸亚铁晶体已氧化变质;若不变红色,则草酸亚铁晶体未氧化变质;
(6)①草酸亚铁晶体的物质的量为:![]() =0.03mol,通过剩余固体的质量为4.32g,则M的摩尔质量为
=0.03mol,通过剩余固体的质量为4.32g,则M的摩尔质量为![]() =144g/mol,过程Ⅰ发生的反应是:草酸亚铁晶体受热失去结晶水,剩下的M为FeC2O4,故答案为:FeC2O4;
=144g/mol,过程Ⅰ发生的反应是:草酸亚铁晶体受热失去结晶水,剩下的M为FeC2O4,故答案为:FeC2O4;
②草酸亚铁晶体中的铁元素质量为:![]() =1.68g,草酸亚铁晶体中的铁元素完全转化到氧化物中,氧化物中氧元素的质量为:
=1.68g,草酸亚铁晶体中的铁元素完全转化到氧化物中,氧化物中氧元素的质量为:![]() ,设铁的氧化物的化学式为FexOy,则有:
,设铁的氧化物的化学式为FexOy,则有:![]() ,解得x:y=3:4,铁的氧化物的化学式为Fe3O4,因此M→N发生反应的化学方程式为3FeC2O4
,解得x:y=3:4,铁的氧化物的化学式为Fe3O4,因此M→N发生反应的化学方程式为3FeC2O4 ![]() Fe3O4+2CO2↑+4CO↑。
Fe3O4+2CO2↑+4CO↑。

【题目】化学与生命活动密切相关。以下是人体中血红蛋白、肌红蛋白与O2结合机制的相关研究,假定其环境温度均为36.8℃。
(1)血红蛋白Hb结合O2形成动脉血,存在反应①:HbH+(aq)+O2(g)![]() HbO2(aq)+H+(aq)。该反应可自发进行,则其ΔH______0(填“>”或“<”);血液中还同时存在反应②:CO2+H2O
HbO2(aq)+H+(aq)。该反应可自发进行,则其ΔH______0(填“>”或“<”);血液中还同时存在反应②:CO2+H2O![]() H++HCO3-,结合反应①②,肺部氧分压_____(填“较高”或“较低”)有利于CO2排出体外,从化学平衡角度解释原因 ____________。
H++HCO3-,结合反应①②,肺部氧分压_____(填“较高”或“较低”)有利于CO2排出体外,从化学平衡角度解释原因 ____________。
(2)肌肉中大量肌红蛋白 Mb也可结合O2形成MbO2,即反应③:Mb(aq)+O2(g)![]() MbO2(aq),其平衡常数K=
MbO2(aq),其平衡常数K=![]() 。其它条件不变,随着氧分压p(O2)增大,K值___(填“变大”、“变小”或“不变”)。已知在氧分压p(O2)=2.00 kPa 的平衡体系中,
。其它条件不变,随着氧分压p(O2)增大,K值___(填“变大”、“变小”或“不变”)。已知在氧分压p(O2)=2.00 kPa 的平衡体系中,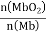 =4.0。吸入的空气中p(O2)=21 kPa,计算此时 Mb与氧气的最大结合度(平衡转化率)约为_______________(保留两位有效数字)。
=4.0。吸入的空气中p(O2)=21 kPa,计算此时 Mb与氧气的最大结合度(平衡转化率)约为_______________(保留两位有效数字)。
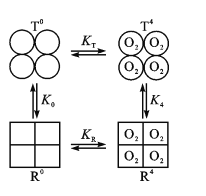
(3)Hb分子具有四个亚基,且每个亚基有两种构型(T型和R型)。图中,T0、R0表示未结合O2的T型和R型,且存在可逆的变构效应:T0![]() R0,正向平衡常数为K0;当四分子O2与Hb的四个亚基结合后,T4
R0,正向平衡常数为K0;当四分子O2与Hb的四个亚基结合后,T4![]() R4也是变构效应,正向平衡常数为K4。
R4也是变构效应,正向平衡常数为K4。
①已知某肺炎病人肺脏中T0+4O2![]() T4反应的n(O2)数据如下:
T4反应的n(O2)数据如下:
t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(O2)/10-6 mol | 1.68 | 1.64 | 1.58 | 1.50 | 1.40 |
计算2.0 min~8.0 min内以T的物质的量变化表示的反应速率v(T4)为_________mol·min-1。
②现假定R型Hb对O2的结合常数为KR,T型Hb对O2的结合常数为KT。已知KR>KT,则图中K0____K4(填“>”或“<”)。
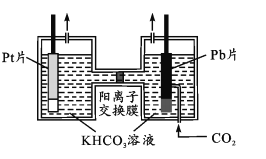
(4)氧气是生命活动必不可少的物质。如下图所示,以Pt为阳极,Pb(CO2)的载体,使CO2活化为阴极,电解经CO2饱和处理的KHCO3溶液可使氧气再生,同时得到甲醇。其阴极反应式为____;从电解后溶液中分离甲醇的操作方法是_______________。
【题目】N2O5是一种新型硝化剂,一定温度下发生2N2O5(g)![]() 4NO2(g)+O2(g) ΔH>0,T1温度下的部分实验数据为
4NO2(g)+O2(g) ΔH>0,T1温度下的部分实验数据为
t/s | 0 | 500 | 1 000 | 1 500 |
c(N2O5)mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
下列说法不正确的是( )
A. 500 s内N2O5分解速率为2.96×10-3 mol/(L·s)
B. T1温度下的平衡常数为K1=125,1 000 s时转化率为50%
C. 其他条件不变时,T2温度下反应到1 000 s时测得N2O5(g)浓度为2.98 mol/L,则T1<T2
D. T1温度下的平衡常数为K1,T2温度下的平衡常数为K2,若T1>T2,则K1>K2