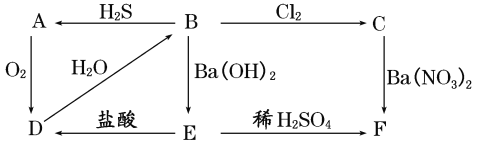
��Ŀ����
����Ŀ���Ե���Ϊ��Ҫԭ�Ϻϳ�һ�־��й���ζ�л���C�߷��ӻ�����E�ĺϳ�·����ͼ1��ʾ��

��ش��������⣺
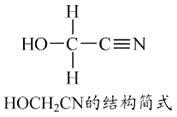
(1)E�Ľṹ��ʽΪ________��D�����ں��еĹ�������________(������)��
(2)д����Ӧ�ڵķ�Ӧ���ͣ�________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��________________________________________________________________________��
��________________________________________________________________________��
(4)ijͬѧ����ͼ2װ���Ʊ�����C���Թ�B��װ�������ı���̼������Һ��Ŀ���ǣ�________________________________________�������Թ�B�ĵ��ܽ���һ����״�������Ϊ________________________________________________________________________�����轫�Թ�B�е�����C����������õ�����Ҫ���������У��ձ���________��

���𰸡�)![]() ̼̼˫�� ������Ӧ 2CH3CH2OH��O2
̼̼˫�� ������Ӧ 2CH3CH2OH��O2![]() 2CH3CHO��2H2O CH3COOH��CH3CH2OH
2CH3CHO��2H2O CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O ���������������ܽ�ȣ����Ҵ������� ������ ��Һ©��
CH3COOCH2CH3��H2O ���������������ܽ�ȣ����Ҵ������� ������ ��Һ©��
��������
CH3CH2OH��Cu���������·�������������AΪCH3CHO��CH3CHO��һ����������BΪCH3COOH��CH3COOH��CH3CH2OH����������Ӧ����CΪCH3COOC2H5���Ҵ�������ȥ��Ӧ����DΪCH2=CH2����ϩ�����Ӿ۷�Ӧ���ɸ߷�������EΪ����ϩ���ݴ˴��⡣
��1�������Ϸ�����֪��EΪ����ϩ���ṹ��ʽΪ��![]() ��DΪ��ϩ���ṹ��ʽΪ��CH2=CH2�����еĹ�����Ϊ̼̼˫�����ʴ�Ϊ��
��DΪ��ϩ���ṹ��ʽΪ��CH2=CH2�����еĹ�����Ϊ̼̼˫�����ʴ�Ϊ��![]() ��̼̼˫����
��̼̼˫����
��2����Ϊ��ȩ��������Ӧ���������ᣬ�ʴ�Ϊ��������Ӧ��
��3����Ϊ�Ҵ��Ĵ�������Ӧ����Ӧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ϊ�������Ҵ���Ũ���������·���������Ӧ����Ӧ����ʽΪ��CH3COOH��CH3CH2OH
2CH3CHO+2H2O����Ϊ�������Ҵ���Ũ���������·���������Ӧ����Ӧ����ʽΪ��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O���ʴ�Ϊ��2CH3CH2OH+O2
CH3COOCH2CH3��H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��CH3COOH��CH3CH2OH
2CH3CHO+2H2O��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
��4���Թ�B��װ�������ı���̼������Һ��Ŀ���Ǣ����������ڱ���Na2CO3��Һ�е��ܽ�Ƚ�С����С�ܽ⣬���ڷֲ��������ڻӷ�����������Na2CO3��Ӧ���������ᣬ�ۻӷ������Ҵ���Na2CO3��Һ���գ��������������ڱ���̼������Һ�����÷�Һ�ķ������룬�õ����������ձ�����Һ©���ȣ������Թ�B�ĵ��ܽ���һ����״�������Ϊ���������ʴ�Ϊ�����������������ܽ�ȣ����Ҵ����������������Һ©����

����Ŀ����֪��25 ��ʱ�й�����ĵ���ƽ�ⳣ�����±���
����Ļ�ѧʽ | CH3COOH | HCN | H2C2O4 |
����ƽ�ⳣ�� | 1.8��10��5 | 4.9��10��10 | K1��5.9��10��2��K2��6.4��10��6 |
�����й�˵����ȷ����(����)
A. CH3COOH��Һ��Na2CO3��Ӧ����CO2����֤������������
B. H2C2O4��Һ�ĵ��뷽��ʽΪ H2C2O4![]() 2H+ + C2O42-
2H+ + C2O42-
C. ��ˮϡ��HCN��Һ���ٽ�HCN�ĵ�����c(CN-)/c(OH-)����
D. ��Na2C2O4 ��Һ�м���������CH3COOH��Һ������Ӧ�����ӷ���ʽΪ C2O42- + CH3COOH=CH3COO- + HC2O4-