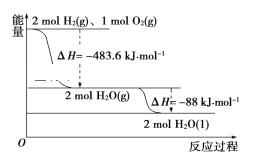
��Ŀ����
����Ŀ����ͼ��ʾ����A��F֮���ת����ϵ������AΪ����ɫ�������ʣ�B��CΪ��ɫ��Һ��DΪ���壬E��FΪ��ɫ����������д���и��գ�
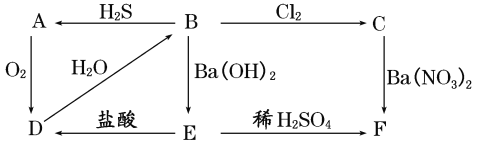
��1��д�������ʵĻ�ѧʽ��
AΪ______��BΪ_____��CΪ_____��DΪ_____��EΪ_____��FΪ______��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��A��________________________��
B��C��___________________________��
��3��д��C��F�����ӷ���ʽ��___________________��
��4����A��F���������У��������������л�ԭ�Ե���(����ĸ����)______��
���𰸡�S H2SO3 H2SO4 SO2 BaSO3 BaSO4 H2SO3��2H2S===3S����3H2O H2SO3��Cl2��H2O===H2SO4��2HCl SO42-��Ba2��===BaSO4�� ABDE
��������
AΪ����ɫ���壬��ת����ϵ��֪��AΪS����DΪSO2����ˮ��Ӧ���ɵ�BΪH2SO3�����л�ԭ�ԣ�������������������ԭ��Ӧ����CΪH2SO4��B������������Ӧ����EΪBaSO3��FΪBaSO4����϶�Ӧ���ʵ����ʷ������
(1)�������Ϸ�����֪��AΪS��BΪH2SO3��CΪH2SO4��DΪSO2��EΪBaSO3��FΪBaSO4���ʴ�Ϊ��S��H2SO3��H2SO4��SO2��BaSO3��BaSO4��
(2)B��A�Ļ�ѧ����ʽ��2H2S+H2SO3=3S��+3H2O��B��C�Ļ�ѧ����ʽ��Cl2+H2SO3+H2O=H2SO4+2HCl���ʴ�Ϊ��2H2S+H2SO3=3S��+3H2O��Cl2+H2SO3+H2O=H2SO4+2HCl��
(3)CΪH2SO4���������ᱵ��Ӧ�������ᱵ��������Ӧ�����ӷ���ʽΪBa2++SO42-�TBaSO4�����ʴ�Ϊ��Ba2++SO42-�TBaSO4����
(4)��A��F���������У��������������л�ԭ�ԣ�˵��SԪ�صĻ��ϼۼ������ߣ����ܽ��ͣ�SԪ�صĻ��ϼ۽����м��̬����S��H2SO3��SO2��BaSO3���ʴ�Ϊ��ABDE��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ����֪Ksp(CaCO3)=2.8��10-9�������й���Ϣ:
���� | CH3COOH | H2CO3 |
����ƽ�ⳣ��(����) | Ka=1.8��10-5 | Ka1=4.3��10-7�� Ka2=5.6��10-11 |
�����ж���ȷ����
A. ��Na2CO3��Һ�е����̪,��Һ��죬��Ҫԭ����CO32��+2H2O![]() H2CO3+2OH��
H2CO3+2OH��
B. ����ʱ��CH3COOH��CH3COONa�����Һ��pH=6����c(CH3COOH)/c(CH3COO-)��18
C. NaHCO3��Һ��:c(OH-)-c(H+)=c(H2CO3)-c(CO32��)
D. 2��10-4 mol/L��Na2CO3��Һ��CaCl2��Һ�������ϳ��ֳ�������CaCl2��Һ��Ũ��һ����5.6��10-5 mol/L
����Ŀ�������Թ��У���ͬ�����·�Ӧ��Fe��2HCl��FeCl2��H2�����жϲ���H2�ķ�Ӧ������С����
�Թ� | ����Ũ�� | �¶� | ����״̬ |
A | 0.5 mol/L | 20 �� | ��״ |
B | 0.5 mol/L | 20 �� | ��ĩ״ |
C | 2 mol/L | 35 �� | ��ĩ״ |
D | 1 mol/L | 35 �� | ��״ |
A. AB. BC. CD. D