��Ŀ����
[��ѧѡ�����ʽṹ������]ͼ1�м��֡�����˹���顱���ü��ڳ�ʽԪ�����ڱ�����ͬ���������⣺
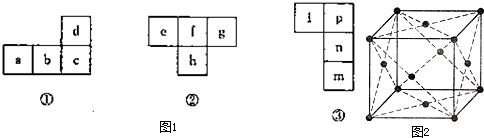
��1��a��d�����ճ������еij���������������d��Ũ�����з����ۻ�����Ԫ��c�����ڱ��е�λ��Ϊ�� ���� �壻 a�ĵ��ʾ����ṹ��ͼ2�����ɾ������֮�������Ϊ ��ÿ�������к��е�ԭ ����Ϊ �������������ÿ��aԭ������ҵȾ����aԭ���� ����
��2��������Ԫ��h����������������������������
����e��f��g�ļ��⻯��ķе��ɸߵ��͵Ĵ���Ϊ�� �� �� ���û�ѧʽ����������ӻ�������ۺͼ۲���ӶԻ������ۣ�h�����Ȼ�����hԭ�ӵ��ӻ�����Ϊ �����ӵĿռ���״Ϊ�� ��
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����ͼ23һ���е�����Ԫ�ؾ��������ڱ��� ��Ԫ�أ�m��n��pԪ�صĵ縺������ �����ǿ������������

��1��a��d�����ճ������еij���������������d��Ũ�����з����ۻ�����Ԫ��c�����ڱ��е�λ��Ϊ��
��2��������Ԫ��h����������������������������
| 1 | 3 |
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����ͼ23һ���е�����Ԫ�ؾ��������ڱ���
��������1��a��d�����ճ������еij�����������Fe��Al��Cu��������d��Ũ�����з����ۻ�����Al��Fe����Ԫ��λ�ù�ϵ��֪��dΪAl����aΪCu��bΪZn��cΪGa��Cu����Ϊ�������壬���þ�̯�����㾧����Cuԭ����Ŀ���Զ���CuΪ�о�������֮�����Cu���������ϣ���ȫ�����ṹ��֪��ÿ������Cuԭ��Ϊ12���湲�ã�
��2��������Ԫ��h����������������������������
����hΪPԪ�أ���Ԫ��λ�ù�ϵ��֪��eΪCԪ�ء�fΪNԪ�ء�gΪOԪ�أ����ݳ�����״̬������ж��⻯��е�ߵͣ�
h�����Ȼ���ΪPCl3������Pԭ�ӵļ۲���Ӷ������¶Ե���ȷ���ӻ���ʽ����ӿռ乹�ͣ�
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����nΪBr����Ԫ��λ�ù�ϵ��֪��lΪS��pΪCl��mΪI����Ԫ�غ�������Ų�������p�ܼ������������ڱ���p��Ԫ�أ�ͬ����Ԫ�����϶��µ縺�Լ�����
��2��������Ԫ��h����������������������������
| 1 |
| 3 |
h�����Ȼ���ΪPCl3������Pԭ�ӵļ۲���Ӷ������¶Ե���ȷ���ӻ���ʽ����ӿռ乹�ͣ�
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����nΪBr����Ԫ��λ�ù�ϵ��֪��lΪS��pΪCl��mΪI����Ԫ�غ�������Ų�������p�ܼ������������ڱ���p��Ԫ�أ�ͬ����Ԫ�����϶��µ縺�Լ�����
����⣺��1��a��d�����ճ������еij�����������Fe��Al��Cu��������d��Ũ�����з����ۻ�����Al��Fe����Ԫ��λ�ù�ϵ��֪��dΪAl����aΪCu��bΪZn��cΪGa����
Ga��Alͬ���壬����Al����һ���ڣ���Ga���ڵ������ڢ�A�壬Cu����Ϊ�������壬���ɾ������֮�������Ϊ����������Cu�����ṹ��֪Ϊ����������������Cu��ĿΪ8��
+6��
=4���Զ���CuΪ�о�������֮�����Cu���������ϣ���ȫ�����ṹ��֪��ÿ������Cuԭ��Ϊ12���湲�ã��ʾ���ÿ��Cuԭ������ҵȾ����Cuԭ����12����
�ʴ�Ϊ���ġ���A����������4��12��
��2��������Ԫ��h����������������������������
����hΪPԪ�أ���Ԫ��λ�ù�ϵ��֪��eΪCԪ�ء�fΪNԪ�ء�gΪOԪ�أ���
e��f��g�ļ��⻯��ֱ�Ϊ CH4��NH3��H2O��������ˮΪҺ�壬�е���ߣ���������֮�����������е�Ƚϼ���ߣ��ʷе�H2O��NH3��CH4��
h�����Ȼ���ΪPCl3��Pԭ�ӵļ۲���Ӷ���=3+
=4���ӻ������Ϊ4��Pԭ�Ӳ���sp3�ӻ���Pԭ����1�Թ¶Ե��ӣ�PCl3���ӵĿռ���״Ϊ�����ͣ�
�ʴ�Ϊ��H2O��NH3��CH4��sp3�������ͣ�
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����nΪBr����Ԫ��λ�ù�ϵ��֪��lΪS��pΪCl��mΪI����
��Ԫ�غ�������Ų�������p�ܼ������������ڱ���p��Ԫ�أ�ͬ����Ԫ�����϶��µ縺�Լ�������m��n��pԪ�صĵ縺�����μ�����
�ʴ�Ϊ��p��������
Ga��Alͬ���壬����Al����һ���ڣ���Ga���ڵ������ڢ�A�壬Cu����Ϊ�������壬���ɾ������֮�������Ϊ����������Cu�����ṹ��֪Ϊ����������������Cu��ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ���ġ���A����������4��12��
��2��������Ԫ��h����������������������������
| 1 |
| 3 |
e��f��g�ļ��⻯��ֱ�Ϊ CH4��NH3��H2O��������ˮΪҺ�壬�е���ߣ���������֮�����������е�Ƚϼ���ߣ��ʷе�H2O��NH3��CH4��
h�����Ȼ���ΪPCl3��Pԭ�ӵļ۲���Ӷ���=3+
| 5-1��3 |
| 2 |
�ʴ�Ϊ��H2O��NH3��CH4��sp3�������ͣ�
��3��n��˫ԭ�ӷ��ӳ�����ΪҺ̬����nΪBr����Ԫ��λ�ù�ϵ��֪��lΪS��pΪCl��mΪI����
��Ԫ�غ�������Ų�������p�ܼ������������ڱ���p��Ԫ�أ�ͬ����Ԫ�����϶��µ縺�Լ�������m��n��pԪ�صĵ縺�����μ�����
�ʴ�Ϊ��p��������
���������������ʽṹ�������ۺ���Ŀ���漰Ԫ���ƶϡ�Ԫ�����ڱ��ṹ�������ṹ�����㡢�ӻ�������۲���ӶԻ������ۡ�Ԫ�������ɵȣ���Ҫѧ���߱���ʵ�Ļ������Ϻÿռ���������ע���������Ԫ�����ڱ��Ľṹ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2011?����һģ����ѧ-ѡ�����ʽṹ�����ʣ�
��2011?����һģ����ѧ-ѡ�����ʽṹ�����ʣ�
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ� [��ѧ--ѡ�����ʽṹ������]�������������Ŀ���ʴ����Խ��Խ�ܵ����ǵ�����������Ҫ����������������ͭ��̼��Ԫ������ɵĺϽ�
[��ѧ--ѡ�����ʽṹ������]�������������Ŀ���ʴ����Խ��Խ�ܵ����ǵ�����������Ҫ����������������ͭ��̼��Ԫ������ɵĺϽ�