��Ŀ����
 ��2011?����һģ����ѧ-ѡ�����ʽṹ�����ʣ�
��2011?����һģ����ѧ-ѡ�����ʽṹ�����ʣ���֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
��1��A�ļ��⻯�������������ԭ�Ӳ�ȡ
sp3
sp3
�ӻ���E�������������ӵĿռ乹����ƽ��������
ƽ��������
����2��B���Ȼ�����۵��D���Ȼ�����۵�
��
��
�����ͣ���������NaClΪ���Ӿ����SiCl4Ϊ���Ӿ���
NaClΪ���Ӿ����SiCl4Ϊ���Ӿ���
����3��A��B��C��D�ĵ�һ��������С�����˳��Ϊ
Na��Al��Si��N
Na��Al��Si��N
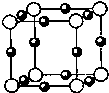
������Ԫ�ط��ű�ʾ����4��A��F�γ�ij�ֻ�����ľ����ṹ����ͼ��ʾ������A��-3�ۣ������仯ѧʽΪ
Cu3N
Cu3N
����ÿ�������ʾ1��ԭ�ӣ���5��F�ĺ�������Ų�ʽ��
1s22s22p63s23p63d104s1����[Ar]3d104s1��
1s22s22p63s23p63d104s1����[Ar]3d104s1��
��A��C�γɵĻ�������и߷е��Ӳ�ȣ���һ���������ǽ������ϣ����仯ѧʽΪAlN
AlN
��������A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F��Aԭ�Ӻ���������δ�ɶԵ��ӣ�������Ų�ʽΪ1s22s22p3����AΪNԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ���CΪAlԪ�أ�Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p63s23p4����EΪSԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬��BΪ�������ڵڢ�A��Ԫ�أ���BΪNaԪ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Si�ľ�������Ϊԭ�Ӿ��壬��������ͬ����DΪSiԪ�أ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ�������������Ų�ʽΪ1s22s22p63s23p63d104s1����FΪCuԪ�أ��Դ������
����⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F��Aԭ�Ӻ���������δ�ɶԵ��ӣ�������Ų�ʽΪ1s22s22p3����AΪNԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ���CΪAlԪ�أ�Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p63s23p4����EΪSԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬��BΪ�������ڵڢ�A��Ԫ�أ���BΪNaԪ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Si�ľ�������Ϊԭ�Ӿ��壬��������ͬ����DΪSiԪ�أ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ�������������Ų�ʽΪ1s22s22p63s23p63d104s1����FΪKԪ�أ�
��1��������Nԭ������1�Թ¶Ե��ӣ��Ҽ���Ϊ3����Nԭ�Ӳ�ȡsp3�ӻ���SO3��Sԭ�Ӳ�ȡsp2�ӻ�����ռ乹��Ϊƽ�������ͣ��ʴ�Ϊ��sp3��ƽ�������ͣ�
��2��B���Ȼ���ΪNaCl��Ϊ���Ӿ��壬��D���Ȼ���ΪSiCl4��Ϊ���Ӿ��壬���Ӿ�����۵���ڷ��Ӿ�����۵㣬�ʴ�Ϊ���ߣ�NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壻
��3���ǽ�����Խǿ����һ������Խ������Խǿ����һ������ԽС����A��B��C��D�ĵ�һ��������С�����˳��ΪNa��Al��Si��N���ʴ�Ϊ��Na��Al��Si��N��
��4��FΪCu��AΪN����NΪ-3�ۣ��ɾ����ṹͼ��֪��Nԭ���ڶ��㣬��Nԭ����Ϊ8��
=1��Cuԭ�������ģ���Cuԭ����Ϊ12��
=3�����Ի�ѧʽΪCu3N���ʴ�Ϊ��Cu3N��
��5��Cu�ĵ����Ų�ʽΪ1s22s22p63s23p63d104s1����[Ar]3d104s1����A��C�γɵĻ�����AlN�����и߷е��Ӳ�ȣ�Ϊԭ�Ӿ��壬
�ʴ�Ϊ��1s22s22p63s23p63d104s1����[Ar]3d104s1����AlN��
��1��������Nԭ������1�Թ¶Ե��ӣ��Ҽ���Ϊ3����Nԭ�Ӳ�ȡsp3�ӻ���SO3��Sԭ�Ӳ�ȡsp2�ӻ�����ռ乹��Ϊƽ�������ͣ��ʴ�Ϊ��sp3��ƽ�������ͣ�
��2��B���Ȼ���ΪNaCl��Ϊ���Ӿ��壬��D���Ȼ���ΪSiCl4��Ϊ���Ӿ��壬���Ӿ�����۵���ڷ��Ӿ�����۵㣬�ʴ�Ϊ���ߣ�NaClΪ���Ӿ����SiCl4Ϊ���Ӿ��壻
��3���ǽ�����Խǿ����һ������Խ������Խǿ����һ������ԽС����A��B��C��D�ĵ�һ��������С�����˳��ΪNa��Al��Si��N���ʴ�Ϊ��Na��Al��Si��N��
��4��FΪCu��AΪN����NΪ-3�ۣ��ɾ����ṹͼ��֪��Nԭ���ڶ��㣬��Nԭ����Ϊ8��
| 1 |
| 8 |
| 1 |
| 4 |
��5��Cu�ĵ����Ų�ʽΪ1s22s22p63s23p63d104s1����[Ar]3d104s1����A��C�γɵĻ�����AlN�����и߷е��Ӳ�ȣ�Ϊԭ�Ӿ��壬
�ʴ�Ϊ��1s22s22p63s23p63d104s1����[Ar]3d104s1����AlN��
���������⿼��ԭ�ӽṹ�����ʣ�Ԫ�ص��ƶ��ǽ����Ĺؼ�����Ϥԭ�ӵĵ����Ų�ʽ���ӻ�����һ�����ܡ������֪ʶ���ɽ���ѶȽϴ�

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ