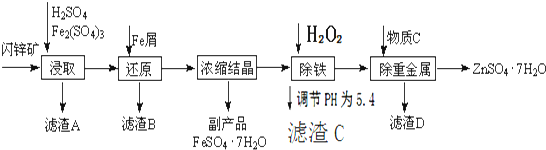
��Ŀ����
17��ijѧУ�о���ѧϰС���ͬѧ����һ���������о�ʵ�����ͬѧ���ռ����ù���������������Ʒ�����ⶨ���к��е���Ҫ���Ӽ������ʵ���Ũ�����£�c��Na+��=1.5��10-4mol/L
c��Mg2+��=4.0��10-5mol/L
c��NH4+��=1.8��10-4mol/L
c��Cl-��=4.0��10-4mol/L
c��SO42-��=1.0��10-5mol/L
�Լ���
��1����������Ʒ��c��H+��=1.0��10-5 mol/L ���������Ӻ��Բ��ƣ���
��2������Һ��pH=5��
���� ��1�����ݵ���غ㣺c��Na+��+2c��Mg2+��+c��NH4+��+c��H+��=c��Cl-��+2c��SO42-����
��2������pH=-lgc��H+�����㣮
��� �⣺��1�����ݵ���غ㣺c��Na+��+2c��Mg2+��+c��NH4+��+c��H+��=c��Cl-��+2c��SO42-������
1.5��10-4mol/L+2��4.0��10-5mol/L+1.8��10-4mol/L+c��H+��=4.0��10-4mol/L+2��1.0��10-5mol/L
��ã�c��H+��=1.0��10-5 mol/L��
�ʴ�Ϊ��1.0��10-5 mol/L��
��2������Һ��pH=-lg1.0��10-5=5���ʴ�Ϊ��5��
���� ���⿼�����ʵ���Ũ�ȼ��㡢��ҺpHֵ���㣬�ѶȲ���ע�����ʻ����Һ�г����õ���غ��������Ũ�ȣ�

��ϰ��ϵ�д�
�����Ŀ
7��������Һһ�������Ե��ǣ�������
| A�� | pH=6.8����Һ | |
| B�� | �����£���ˮ�����OH-����Ũ��Ϊ1��10-13 mol/L | |
| C�� | �����̪�����Ժ�ɫ����Һ | |
| D�� | �����£���Һ�е�H+����Ũ��Ϊ5��10-7 mol/L |
8������˵����ȷ���ǣ�������
| A�� | ��Na2CO3��Һ�У�c��Na+����c��CO32-��=2��1 | |
| B�� | ��25��ʱ�����������ԡ����Ի�������Һ�У���c��H+����c��OH-���ij˻�������1��10-14 | |
| C�� | 0.1mol/L��KOH��Һ��0.1mol/L��ˮ�У�c��OH-����� | |
| D�� | ԭ��ص������������ķ�Ӧ��������Ӧ |
5�����и���������ȫ����������ʵ��ǣ�������
| A�� | NH3��Cl2��H3PO4 | B�� | AgCl��Ba��OH��2��H2S | ||
| C�� | H2SO3��NaOH��H2SO4 | D�� | NH3•H2O��H2O��CH3COOH |
12����pH=1��ij��Һ�У�����������ԭ��Ӧ�����ܴ���������������ǣ�������
| A�� | A13+��NO3-��Fe2+ | B�� | SCN-��Cl-��Fe3+ | C�� | HCO3-��Al3+��Cl- | D�� | Na+��K+��CO32- |
2������������ȷ���ǣ�������
| A�� | ���ܸ��ᷴӦ��������һ���ܸ��Ӧ | |
| B�� | ͬһ����Ԫ�ص��⻯���Է�������Խ�����ķе�һ��Խ�� | |
| C�� | ��CCl4��PCl3��HCl�и�ԭ���������ܴﵽ8�����ȶ��ṹ | |
| D�� | �����¶ȿ��Լӿ췴Ӧ���ʣ�����Ҫԭ���Ƿ�Ӧ������������ӣ�����Ӱٷ��������ӣ�ʹ��Ч��ײ�������� |
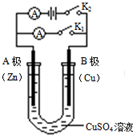 ����ͼ��ʾ���밴Ҫ��ش��������⣮
����ͼ��ʾ���밴Ҫ��ش��������⣮