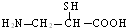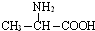��Ŀ����
6��ƻ����㷺������ˮ�����У���һ�ֳ��õ�ʳƷ���Ӽ��������������ƻ�������Է�������Ϊ134������5�ֲ�ͬ������Hԭ�ӣ��أ�C��=35.82%���أ�H��=4.48%���أ�O��=��59.70%��ȡ2.68gƻ��������ˮ�����Һ����0.80mol/LNaOH��Һ�ζ���ȥ50.00mLǡ���кͣ�1molƻ������������Na��Ӧ����1.5mol��H2������
��1��ƻ����ķ���ʽ��
��2��ƻ��������к��Ȼ��ĸ���
��3����д��ƻ����Ľṹ��ʽ��
���� ��1������ƻ�������Է�����������Ԫ�ص�������������������ʽ��
��2������n=$\frac{m}{M}$�����ƻ��������ʵ���������n=cV������������Ƶ����ʵ�����Ȼ����ݸ��ݹ�ϵʽ��-COOH��x��xNaOH�����ƻ�����к����Ȼ�����Ŀ��
��3������ƻ����ķ���ʽ����1molƻ������������Na��Ӧ����1.5mol��H2����������5�ֲ�ͬ������Hԭ�ӡ�ȷ����ṹ��ʽ��
��� �⣺��1��ƻ��������к�CΪ��N��C��=$\frac{134��35.82%}{12}$=4����HΪ��N��H��=$\frac{134��4.48%}{1}$=6������Ϊ��N��O��$\frac{134��59.70%}{16}$=5���������ʽΪ��C4H6O5��
��ƻ����ķ���ʽΪC4H6O5��
��2��2.68gƻ��������ʵ���Ϊ��$\frac{2.68g}{134g/mol}$=0.02mol��
50.00mL 0.8mol/L��NaOH��Һ�к����������Ƶ����ʵ���Ϊ��0.80mol/L��0.050L=0.04mol��
��ƻ������x��-COOH�����ݹ�ϵʽ��-COOH��x��xNaOH��֪��x=$\frac{0.04mol}{0.02mol}$=2��
��ƻ�����к����Ȼ�����ĿΪ2��
��3��1molƻ������������Na��Ӧ����1.5mol��H2��˵�������к���3��-OH������-COOH�������������Ȼ�������1���ǻ���
ƻ�����������ԭ�Ӵ���5�ֲ�ͬ�Ļ�ѧ������˵����5�ֲ�ͬ��Hԭ�ӣ���ƻ����Ľṹ��ʽΪ��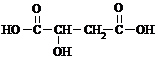
��ƻ����Ľṹ��ʽΪ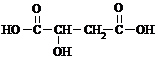
���� ���⿼�����л���ṹ��ʽ���ṹʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ�����3��Ϊ�״��㣬��Ҫ�ۺ�ƻ����ķ���ʽ����1molƻ������������Na��Ӧ����1.5mol��H2����������5�ֲ�ͬ������Hԭ�ӡ�ȷ����ṹ��

| A�� | �����¶� | B�� | ������Ƭ������ | ||
| C�� | �����۴�����Ƭ | D�� | ��98%��Ũ�������ϡ���� |
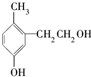
| A�� | ���DZ��ӵ�ͬϵ�� | |
| B�� | 1mol���л�������2 mol��ˮ����ȡ����Ӧ | |
| C�� | 1mol���л�����������Ʒ�Ӧ����0.5 mol H2 | |
| D�� | 1mol���л�������2 mol NaOH��Ӧ |
| A�� | 598 | B�� | 576 | C�� | 288 | D�� | 299 |
| A�� | �����¶�ֻ����淴Ӧ���� | B�� | �����¶������淴Ӧ���ʶ����� | ||
| C�� | ����ֻ������Ӧ���� | D�� | ��Сѹǿʱֻ���淴Ӧ���ʽ��� |
| A�� | 0.1mol•L-1•S-1 | B�� | 0.4 mol•L-1•S-1 | C�� | 0.2 mol•L-1•S-1 | D�� | 0.6mol•L-1•S-1 |
 ��
�� ��E���ӵĵ����Ų�ʽΪ1s22s22p6��Bԭ�ӵĵ����Ų�ͼΪ
��E���ӵĵ����Ų�ʽΪ1s22s22p6��Bԭ�ӵĵ����Ų�ͼΪ ��
��