题目内容
【题目】草酸是一种二元弱酸,可用作还原剂、络合剂、掩蔽剂、沉淀剂。某校课外小组的同学设计利用C2H2气体制取H2C2O4·2H2O。
回答下列问题:
(1)甲组的同学利用电石(主要成分CaC2,少量CaS及Ca3P2等)并用下图装置制取C2H2[反应原理为:CaC2+2H2O![]() Ca(OH)2+C2H2(g) △H<0,反应剧烈]:
Ca(OH)2+C2H2(g) △H<0,反应剧烈]:
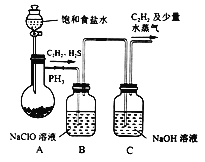
① 装置A用饱和食盐水代替水并缓慢滴入烧瓶中,其目的是__________。
② 装置B中,NaClO将H2S、PH3氧化为硫酸及磷酸,本身还原为NaCl,其中H2S被氧化的离子方程式为________。
(2)乙组的同学根据文献资料,用Hg(NO3)2作催化剂,浓硝酸氧化乙炔制取H2C2O4·2H2O。制备装置如下:
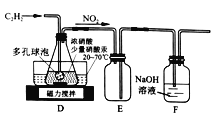
①装置D多孔球泡的作用是_________;装置E的作用是________。
②D中生成H2C2O4的化学方程式为_______。
③从装置D得到产品,还需经过浓缩结晶、________(填操作名称)洗涤及干燥。
(3)丙组设计了测定乙组产品在H2C2O4·2H2O的质量分数实验。他们的实验步骤如下:精确称取m g产品于锥形瓶中,加入适量的蒸馏水溶解,在加入少量稀硫酸,然后用cmol/L酸性KMnO4标准溶液进入滴定,滴至溶液显微红色;共消耗标准溶液VmL。
①滴定时,KMnO4标准溶液盛放在__________滴定管中(填“酸式”或“碱式”)。
②滴定时KMnO4被还原Mn2+,其反应的离子方程式为_____,滴定过程中发现褪色速率先慢后逐渐加快,其主要原因是__________。
③产品中H2C2O4·2H2O的质量分数为_________(列出含m、c、v的表达式)。
【答案】 减慢反应速率,平缓地产生乙炔 H2S + 4ClO-= SO42-+ 4Cl-+2H+ 增大乙炔气体与硝酸的接触面,充分反应 防止倒吸 C2H2+8HNO3![]() H2C2O4+8NO2+4H2O 过滤 酸式 2MnO4++5H2C2O4 +6H+ = 2Mn2++10CO2↑+8H2O 生成的Mn2+是该反应的催化剂
H2C2O4+8NO2+4H2O 过滤 酸式 2MnO4++5H2C2O4 +6H+ = 2Mn2++10CO2↑+8H2O 生成的Mn2+是该反应的催化剂 ![]()
【解析】试题分析:(1)饱和食盐水代替水能减缓反应速率。
②NaClO与H2S反应生成硫酸和NaCl。
(2)①多孔球泡能增大气体与液体的接触面;装置E能防倒吸。
②硝酸与乙炔反应生成H2C2O4和NO2。
③从装置D得到产品,还需经过浓缩结晶、过滤、洗涤及干燥。
(3)①KMnO4标准溶液具有强氧化性,能腐蚀橡胶。
②滴定时KMnO4被还原Mn2+,H2C2O4被氧化为CO2,根据影响反应速率的因素分析滴定过程中发现褪色速率先慢后逐渐加快的主要原因。
③根据化学方程式计算产品中H2C2O4·2H2O的质量分数。
解析:根据以上分析,(1)饱和食盐水代替水能减缓反应速率,平缓地产生乙炔。
②NaClO与H2S反应生成硫酸和NaCl,反应离子方程式为H2S + 4ClO-= SO42-+ 4Cl-+2H+。
(2)①多孔球泡能增大气体与液体的接触面,提高反应速率及原料利用率;装置E气体短进长处,所以能防倒吸。
②硝酸与乙炔反应生成H2C2O4和NO2,反应方程式为C2H2+8HNO3![]() H2C2O4+8NO2+4H2O。
H2C2O4+8NO2+4H2O。
③从装置D得到产品,还需经过浓缩结晶、过滤、洗涤及干燥。
(3)①KMnO4标准溶液具有强氧化性,能腐蚀橡胶,KMnO4标准溶液盛放在酸式滴定管中。
②滴定时KMnO4被还原Mn2+,H2C2O4被氧化为CO2,反应离子方程式为2MnO4++5H2C2O4 +6H+ = 2Mn2++10CO2↑+8H2O,根据影响反应速率的因素,滴定过程中发现褪色速率先慢后逐渐加快的主要原因是生成的Mn2+是该反应的催化剂。
③设产品中H2C2O4·2H2O的物质的量为x mol。
2MnO4+ + 5H2C2O4 + 6H+ = 2Mn2++10CO2↑+8H2O
2 5
VmL![]() cmol/L x
cmol/L x
![]()
X=2.5VC![]() ,质量分数为
,质量分数为![]()

 阅读快车系列答案
阅读快车系列答案