题目内容
【题目】(1)铁及其化合物在生活、生产中有广泛的应用。请回答下列问题:
①黄铁矿(FeS2)是生产硫酸和冶炼钢铁的重要原料。其中一个反应为3FeS2+8O2=6SO2+Fe3O4,氧化产物为_____,若有3mol FeS2参加反应,转移__________mol电子。
②FeCl3与氢碘酸反应时可生成棕色物质I2,该反应的离子方程式为_________________。
(2)已知:S2O32-具有较强的还原性,实验室可用I-测定测定K2S2O8样品的纯度:反应方程式为:
S2O82-+2I-→2SO42-+I2,I2+2S2O32-→2I-+S4O62-则S2O82-、S4O62-、I2氧化性强弱顺序:_________.
(3) 已知溶液中:还原性HSO3->I-,氧化性IO3-> I2 > SO42-。在含3 molNaHSO3的溶液中逐滴加入KIO3溶液,加入的KIO3和析出的I2的物质的量的关系曲线如图所示。
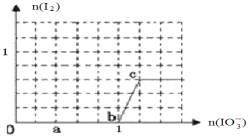
①写出a点处还原产物是_______________;b点到c点反应的离子方程式_________________________。
②当溶液中的I-为0.5 mol时,加入的KIO3为___________mol。
【答案】SO2、Fe3O4322Fe3++2I-=2Fe2++I2S2O82->I2>S4O62-I-IO3-+5I-+6H+=3I2+3H2O0.5或1.1
【解析】
(1)①在反应3FeS2+8O2![]() 6SO2+Fe3O4中,FeS2中Fe、S元素的化合价升高,失去电子,被氧化,O2中的O元素的化合价降低,被还原,所以氧化产物为SO2、Fe3O4;3molFeS2参加反应,由O元素的化合价变化可知,转移的电子为8mol×2×(2-0)=32mol;②FeC13与氢碘酸反应时可生成棕色物质是I2,根据电子守恒、电荷守恒及原子守恒,可得该反应的离子方程式为2Fe3++2I-=2Fe2++I2;(2)在氧化还原反应中氧化性:氧化剂>氧化产物,由S2O82-+2I-=2SO42-+I2可知氧化性:S2O82->I2;由I2+2S2O32-=2I-+S4O62-可知氧化性:I2>S4O62-.所以氧化性:S2O82->I2>S4O62-;(3)HSO3->I-,在含3 mol NaHSO3的溶液中逐滴加入KIO3溶液,首先发生反应:3HSO3-+IO3-=3SO42-+I-+3H+;HSO3-反应完毕,继续加入KIO3,由于氧化性IO3->I2,再发生反应:IO3-+6H+ +5I- =3H2O+3I2。①由图可知,a点没有生成碘,故发生反应3HSO3-+ IO3-=3SO42-+I-+3H+,反应中S元素化合价升高,还原剂是HSO3-,I元素的化合价降低,I元素被还原,所以此处的还原产物是I-,由图可知,b点到c点由于生成I2,故发生反应:IO3-+6H+ +5I-=3H2O+3I2。②当溶液中的I-为0.5mol时,有两种情况:一是只发生反应3HSO3-+IO3-=3SO42-+I-+3H+,生成I-为0.5mol,根据碘元素守恒n(KIO3) =n(I-)=0.5mol;二是HSO3-反应完毕后,还发生IO3-+6H+ +5I-=3H2O+3I2,剩余I-为0.5mol,3molNaHSO3消耗1molKIO3、生成1molI-,故反应IO3-+6H+ +5I- =3H2O+3I2中消耗的I-为1mol-0.5mol=0.5mol,消耗KIO3的物质的量为0.5mol×1/5=0.1mol,故共加入的KIO3为1mol+0.1mol=1.1mol。
6SO2+Fe3O4中,FeS2中Fe、S元素的化合价升高,失去电子,被氧化,O2中的O元素的化合价降低,被还原,所以氧化产物为SO2、Fe3O4;3molFeS2参加反应,由O元素的化合价变化可知,转移的电子为8mol×2×(2-0)=32mol;②FeC13与氢碘酸反应时可生成棕色物质是I2,根据电子守恒、电荷守恒及原子守恒,可得该反应的离子方程式为2Fe3++2I-=2Fe2++I2;(2)在氧化还原反应中氧化性:氧化剂>氧化产物,由S2O82-+2I-=2SO42-+I2可知氧化性:S2O82->I2;由I2+2S2O32-=2I-+S4O62-可知氧化性:I2>S4O62-.所以氧化性:S2O82->I2>S4O62-;(3)HSO3->I-,在含3 mol NaHSO3的溶液中逐滴加入KIO3溶液,首先发生反应:3HSO3-+IO3-=3SO42-+I-+3H+;HSO3-反应完毕,继续加入KIO3,由于氧化性IO3->I2,再发生反应:IO3-+6H+ +5I- =3H2O+3I2。①由图可知,a点没有生成碘,故发生反应3HSO3-+ IO3-=3SO42-+I-+3H+,反应中S元素化合价升高,还原剂是HSO3-,I元素的化合价降低,I元素被还原,所以此处的还原产物是I-,由图可知,b点到c点由于生成I2,故发生反应:IO3-+6H+ +5I-=3H2O+3I2。②当溶液中的I-为0.5mol时,有两种情况:一是只发生反应3HSO3-+IO3-=3SO42-+I-+3H+,生成I-为0.5mol,根据碘元素守恒n(KIO3) =n(I-)=0.5mol;二是HSO3-反应完毕后,还发生IO3-+6H+ +5I-=3H2O+3I2,剩余I-为0.5mol,3molNaHSO3消耗1molKIO3、生成1molI-,故反应IO3-+6H+ +5I- =3H2O+3I2中消耗的I-为1mol-0.5mol=0.5mol,消耗KIO3的物质的量为0.5mol×1/5=0.1mol,故共加入的KIO3为1mol+0.1mol=1.1mol。

【题目】下列实验、现象及有关结论不正确的是
选项 | A | B | C | D |
实验 |
|
|
|
|
现象 | 加热铝箔,铝箔熔化却不滴落 | 石蜡油分解产生的气体能使试管中溴的四氯化碳溶液褪色 | 食盐水浸泡过的铁钉放入试管中,一段时间后,导管口形成一段水柱 | 向蔗糖中加人浓硫酸时,蔗糖变黑,体积膨胀 |
结论 | 氧化铝的熔点比铝的高 | 石蜡油的分解产物中含不饱和烃 | 铁钉发生吸氧腐蚀 | 浓硫酸具有吸水性和强氧化性 |
A. A B. B C. C D. D
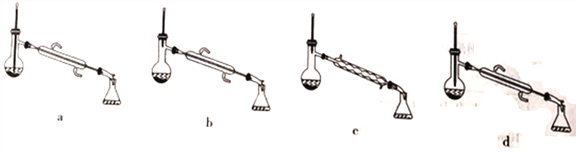
【题目】工业上用过量的乙酸和异成醇制备乙酸异戊酯,原理(如图所示) 和有关数据如下:
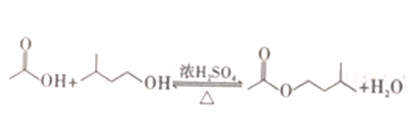
物质 | 相对分子质量 | 密度/(g·cm-3) | 熔点/℃ | 沸点/℃ | 水中溶解度 |
异戊醇 | 88 | 0.8123 | -117 | 131 | 微溶 |
乙酸 | 60 | 1.0492 | 17 | 118 | 溶 |
乙酸异戊酯 | 130 | 0.8670 | -78 | 142 | 难溶 |
下列说法正确的是
A. 常温下,可用过滤的方法分离乙酸异戊酯和水溶液
B. 反应中,加入过量的乙酸的主要目的是加快该反应的反应速率
C. 为了除去产物中混有的乙酸,可以向混合物中加入足量饱和Na2CO3溶液,充分振荡后静置、分液
D. 为了除去产物中混有的异戊醇,应选择如图所示装置中的c装置