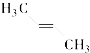题目内容
【题目】以含钴废催化剂(主要成分为Co、Fe、SiO2)为原料,制取氧化钴的流程如下。
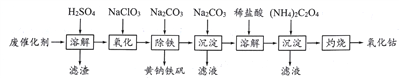
(1)溶解:溶解后过滤,将滤渣洗涤2~3次,洗液与滤液合并,其目的是__________________。
(2)氧化:加热搅拌条件下加入NaC1O3,其作用是_______________________________。
(3)除铁:加入适量的Na2CO3调节酸度,生成黄钠铁矾Na2[Fe6(SO4)4(OH)12]沉淀。写出该反应的化学方程式:__________________________________________________。
(4)沉淀:生成沉淀碱式碳酸钴[(CoCO3)2·3Co(OH)2],沉淀需洗涤,检验沉淀是否洗涤干净的操作是_________________________________________________________。
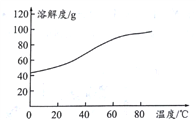
(5)溶解:CoCl2的溶解度曲线如图所示。向碱式碳酸钴中加入足量稀盐酸,边加热边搅拌至完全溶解后,需趁热过滤,其原因是__________________________________。
(6)灼烧:准确称取所得CoC2O4固体2.205g,在空气中灼烧得到钴的一种氧化物1.205g,写出该氧化物的化学式_________________________。
【答案】 提高钴元素的利用率(或减少钴的损失) 将Fe2+氧化成Fe3+ 3Fe2(SO4)3+6H2O+6Na2CO3=Na2Fe6(SO4)4(OH)12↓+5Na2SO4+6CO2↑(写成离子方程式不扣分) 取最后洗涤液少许于试管中,滴加几滴硝酸银溶液,若无白色沉淀生成,则已洗净,否则没有洗净 防止因温度降低而析出CoCl2晶体 Co3O4
【解析】本题考查工艺流程。主要涉及的考点为化学方程的书写、离子的检验、沉淀的洗涤、对图象的分析处理等。(1)将滤渣洗涤2~3次,洗液与滤液合并,提高钴元素的利用率;(2)加入NaC1O3,亚铁离子被氯酸根离子氧化成铁离子,其反应为:可知离子方程式为:6Fe2++6H++ClO3-═6Fe3++Cl-+3H2O;(3) 生成硫酸铁与碳酸钠发生双水解得到黄钠铁矾,化学反应方程式为:3Fe2(SO4)3+6H2O+6Na2CO3=Na2Fe6(SO4)4(OH)12↓+5Na2SO4+6CO2↑;(4)洗涤的目的是洗去固体表面可溶性杂质,如:Cl—、SO42—等。检验沉淀是否洗涤干净的操作是:取最后洗涤液少许于试管中,滴加几滴硝酸银溶液,若无白色沉淀生成,则已洗净,否则没有洗净;(5) CoCl2的溶解度曲线可知,随温度的升高,CoCl2的溶解度增大,所以趁热过滤,防止温度降低氯化钴析出;(6) CoC2O4的质量为2.205g,n(CoC2O4)=![]() =0.015mol,该氧化物中m(Co)=0.015 mol×59 g·mol-1=0.885 g,m(O)=1.205g g-0.885 g=0.32 g,n(Co)∶n(O)=0.015mol∶
=0.015mol,该氧化物中m(Co)=0.015 mol×59 g·mol-1=0.885 g,m(O)=1.205g g-0.885 g=0.32 g,n(Co)∶n(O)=0.015mol∶![]() =3∶4,故氧化钴化学式为Co3O4。
=3∶4,故氧化钴化学式为Co3O4。

【题目】根据下表中的信息分析回答问题:
元素 | 元素或由元素组成的物质性质 |
A | 单质在自然界中硬度最大,燃烧产生的气体能使澄清石灰水变浑浊。 |
B | 原子最外层电子数是次外层电子数三倍 |
C | 金属焰色反应为黄色,与氧气燃烧生成淡黄色固体 |
D | 单质在空气中体积分数最大 |
E | 固体为淡黄色,燃烧产生气体有漂白性。 |
F | 相同条件下气体密度最小 |
(1)用电子式写出形成F2E的过程__________________;
(2)写出CBF物质的电子式______________;
(3)C2B2物质中存在化学键的类型是___________ ,1 molC2B2与足量AB2反应转移的电子数为_______;
(4)由B、C、E形成的简单离子半径由大到小的关系是______________(用离子符号表示)。