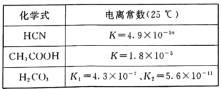
��Ŀ����
����Ŀ����������ͼʾ���ý�����ȷ����
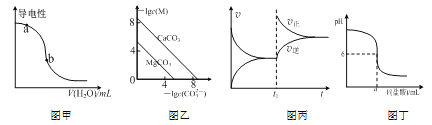
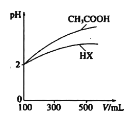
A.ͼ�ױ�ʾһ���¶��£�CH3COOHϡ��Һ��ˮʱ��Һ�ĵ����Ա仯����pH��a����pH��b��
B.ͼ�ұ�ʾһ���¶��£�MCO3��M��Mg2+��Ca2+���ij����ܽ�ƽ�����ߣ���Ksp��MgCO3����Ksp��CaCO3��
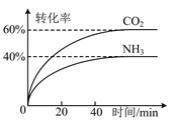
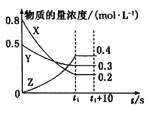
C.ͼ����ʾһ���¶��£����淴ӦN2��g����3H2��g�� ![]() 2NH3��g�����淴Ӧ������ʱ��仯�����ߣ�t1ʱ�ı�����������Ƿ����NH3
2NH3��g�����淴Ӧ������ʱ��仯�����ߣ�t1ʱ�ı�����������Ƿ����NH3
D.ͼ����ʾ�����½�0.1 mol��L-1������Һ�μӵ�a mL0.1 mol��L��1BOH��Һ�У�������ҺpH��������������Һ����ı仯ͼ����BOHΪ����
���𰸡�D
��������
A. ����ͼ����ʾ��CH3COOHϡ��Һ��ˮʱ��Һ�ĵ����Լ�����˵������Ũ���ڼ�С������c(H+)(a)��c(H+)(b)����pH(a)��pH(b)��Aѡ�����
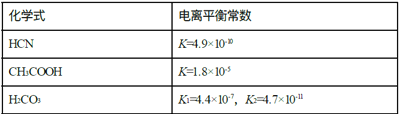
B. �۲�ͼ��֪��-lgc(CO32-)��ȼ�c(CO32-)���ʱ��-lgc(Mg2+)��-lgc(Ca2+)����c(Mg2+)��c(Ca2+)�����ԣ�Ksp(MgCO3)��Ksp(CaCO3)��Bѡ�����
C. �����NH3������Ũ�Ƚ��ͣ��淴Ӧ���ʽ���С��Cѡ�����
D. ͼ�����к͵ζ����ߣ��۲�ͼ��֪��ǡ����ȫ��Ӧʱ����Һ�����ԣ�˵����Ӧ���ɵ���ǿ�������Σ�����BOHΪ���Dѡ����ȷ��
��ѡD��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����Ŀ������ʵ���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
|
|
|
|
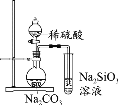
A���Ʊ����ռ��������� | B��֤���Ȼ����ܽ�ȴ������� | C����֤���������ȥ��������ϩ | D���ƶ�S��C��Si�ķǽ�����ǿ�� |
A.AB.BC.CD.D