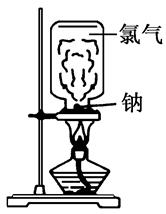
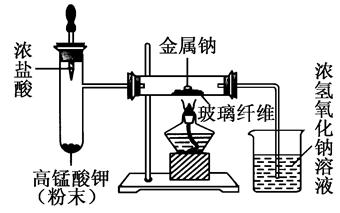
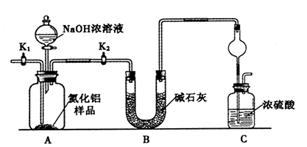
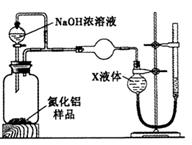
��Ŀ����
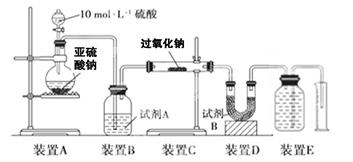
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ�2��3�Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼���Ƶ������������ס��ҡ�����λͬѧ�ֱ����������ʵ�鷽����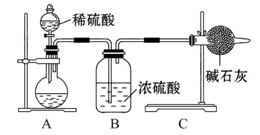
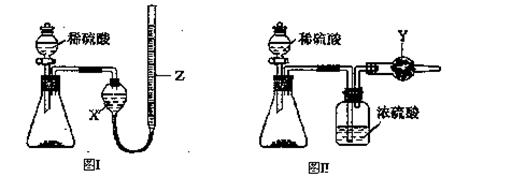
��ͬѧ�ķ�����ͼ��ʾ��
��1����μ���Aװ�õ������ԣ�_____________________________________________��
��2����ͬѧ�ظ�����������ʵ�飬�õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊ��������������ƫ�͵�ԭ����_______������ţ���
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㡢��Ӧ�����
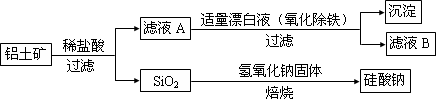
��3��Ϊ���ü�ʵ����������ȷ��������ʵ�鲽�趼��ȷ�������£�����Ϊͼ�е�ʵ��װ��Ӧ����θĽ���______________��
����ͬѧ�ķ����ǣ���ͼ�����ṩ��װ����ѡ��ʵ��װ�ã������ͬѧʵ��װ���е�B��C��ͨ���ⶨ�ų��Ķ�����̼������������Ƕ�����̼����ˮ�������㡣
ѡ�����װ�õ�����˳��Ϊ_______��
��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӡ���ɡ��������ù���n g��
��1������100 mL 0��10 mol/L BaCl2��Һ��ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ���_______�����������ƣ���
��2���������̼���Ƶ���������Ϊ����m��n��ʾ��_______��
��3��Ca2+��Ba2+������ʹ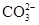 ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
��1����ֹˮ�мн�Aװ�õ�����ĩ�˵���Ƥ�ܣ���Һ©���ϲ������ӣ�������ע����������ˮ����������ʼ������ˮ���£���һ�����ˮ���ܵ���Բ����ƿ��֤��װ������������
��2��C��D ��3����װ��ʯ�ҵĸ�����ұ���װһ��ʢ�м�ʯ�ҵĸ���ܣ���ֹ������ˮ�ֺͶ�����̼��Cװ���еļ�ʯ������ �ݢߢ�
��1��100 mL����ƿ
��2��106n/197m
��3���� ������Ca2+����OH-��������ˮ���������Ƴ�����Ӱ����
����

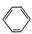
��12�֣�����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ��������������в�����Ԫ�أ������ʲ���H2��H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
������ʯ�к������IJⶨ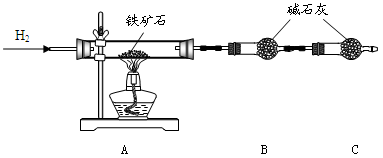
ʵ��������5.0g����ʯ����Ӳ�ʲ���������ȫ��Ӧ�� ���װ��B����1.35 g��
������ʯ�к������IJⶨ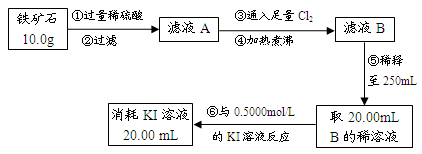
����������������
��1����Ҫ��֤��ҺA����Ԫ�صĴ�����ʽ������ȡ������ҺA�ֱ����ʵ�飬ʵ�鷽����������������±����뽫�䲹��������
�ɹ�ѡ����Լ��У�����KMnO4��Һ��NaOH��Һ��KSCN��Һ����ˮ
| ʵ�鷽�� | ʵ������ | ���� |
| ����1������ҺA�м��� | | ��ҺA����Fe3�� |
| ����2������ҺA�м��� | | ��ҺA����Fe2�� |
��3��������з�Ӧ�����ӷ���ʽΪ�� ��
��4����ͨ��ʵ�������������ʯ������������Ļ�ѧʽ����д��������̣�
������������(FeC2O4��2H2O)���������Լ�����Ӱ�������͵�ز�����������﮵��������ش��������⣺
I����ȤС��Բ�����������ķֽ�������ʵ���̽����̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
��1���������
����һ��___________�� �������ȫ����FeO �� ��������FeO��Fe����
��2�����ʵ�鷽��֤����������
| ʵ�鲽�� | ��������� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı࣬���� ���ɣ���֤���������ʴ��� |
| ����2��������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȥ����2�õ������������Թ��У��μ� | |
��ѡ�Լ���ϡ���ᡢ���Ƶ���ˮ��0.1mol��L-1CuSO4��Һ��20% KSCN��Һ������ˮ��
����ȤС���������в��ĵ���FeC2O4��2H2O���ȷֽ�ʱ�������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��_______________
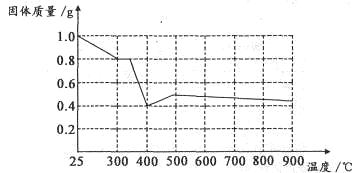
����ͼ������1.0g�������������������г��ڳ�ּ��ȣ����ղ�����ɫ�������������0.4g��ijͬѧ�ɴ˵ó����ۣ�����������������Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ�______________________��
ij̽��С�������ͼ��ʾװ�ý���Fe����ˮ�����ķ�Ӧ��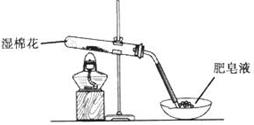
��1��ʵ��ǰ���װ�������Եķ���Ϊ________________________________________________________��
��2������ʵ�������������ʵ�������_____________________________________________��
��3����̽��С���Ϊ���飬����ͼװ�ý��жԱ�ʵ�飬�����þƾ���ơ������þƾ��Ƽ��ȣ���Ӧ�����Ϊ��ɫ��ĩ(������)������ֱ��ò����������ʵ�顣
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�����KMnO4��Һ | ��ɫ��ȥ | ��ɫ��ȥ |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
������õ��ĺ�ɫ��ĩ�� ��
�ڼ��鲽��1�з�Ӧ�����ӷ���ʽΪ ��
�����鲽��4�У���Һ����ԭ��Ϊ ����Һ��ɫ���ܵ�ԭ���� ����֤����Ϊ ��