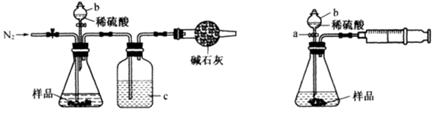
��Ŀ����
������������(FeC2O4��2H2O)���������Լ�����Ӱ�������͵�ز�����������﮵��������ش��������⣺
I����ȤС��Բ�����������ķֽ�������ʵ���̽����̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
��1���������
����һ��___________�� �������ȫ����FeO �� ��������FeO��Fe����
��2�����ʵ�鷽��֤����������
| ʵ�鲽�� | ��������� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı࣬���� ���ɣ���֤���������ʴ��� |
| ����2��������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȥ����2�õ������������Թ��У��μ� | |
��ѡ�Լ���ϡ���ᡢ���Ƶ���ˮ��0.1mol��L-1CuSO4��Һ��20% KSCN��Һ������ˮ��
����ȤС���������в��ĵ���FeC2O4��2H2O���ȷֽ�ʱ�������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��_______________
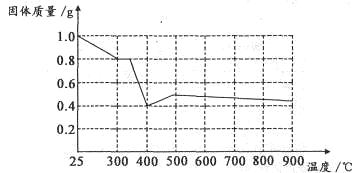
����ͼ������1.0g�������������������г��ڳ�ּ��ȣ����ղ�����ɫ�������������0.4g��ijͬѧ�ɴ˵ó����ۣ�����������������Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ�______________________��
��1��ȫ����Fe
��2��
II��FeC2O4��2H2Oʵ�鲽�� ��������� ����1������ͭ��Һ (��)��ɫ���� ����3������HCl�����ã�ȡ�ϲ���Һ���μӼ���KSCN��Һ���ٵμ��������Ƶ���ˮ ,����� ���μ��������Ƶ���ˮ����Һ�ʺ�ɫ����֤����FeO  FeO+CO��+CO2��+2H2O
FeO+CO��+CO2��+2H2O
��ͬ�⣬ʵ��û�����ܱ������н��У�FeO�ᱻ�����е�������һ��������������������������
�������������
��1���������ʵ���ɼ�����ļ������������֪����һΪ����ȫ����Fe����2������һ���������к���FeO��Fe.�����ڽ���Fe�DZȽϻ��õĽ������ܰѻ�Ա������Ľ����û����������Կ�������������м�������ͭ��Һ��������ã���������Һ����ɫ��dz��ͬʱ��������ɫ�Ĺ��塣��֤������Fe���ʡ�������������������õĵĹ�����뵽������ϡHCl�У�������Ӧ��FeO+2HCl=FeCl2+H2O.������ã�ȡ�ϲ���Һ���μӼ���KSCN��Һ���������ٵμӼ������Ƶ���ˮ ,�������������Һ��ΪѪ��ɫ����֤�������к���FeO.
II ���������غ㶨�ɺ͵����غ�Ĺ��ɿ�֪FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪFeC2O4��2H2O FeO+CO��+CO2��+2H2O�����ݷ���ʽFeC2O4��2H2O
FeO+CO��+CO2��+2H2O�����ݷ���ʽFeC2O4��2H2O FeO+CO��+CO2��+2H2O��֪1g����FeC2O4��2H2O�ֽ������FeO������Ϊ0.4g.�����ڷֽⷴӦ�������г��ڳ�ּ��ȣ�û�����ܱյ������ڽ��С�FeO�л�ԭ�ԣ��ڼ���ʱ���ױ������е���������ΪFe2O3.���Թ���������ܴ���0.4g.��˲����ɴ˵õ�����˵�������������
FeO+CO��+CO2��+2H2O��֪1g����FeC2O4��2H2O�ֽ������FeO������Ϊ0.4g.�����ڷֽⷴӦ�������г��ڳ�ּ��ȣ�û�����ܱյ������ڽ��С�FeO�л�ԭ�ԣ��ڼ���ʱ���ױ������е���������ΪFe2O3.���Թ���������ܴ���0.4g.��˲����ɴ˵õ�����˵�������������
���㣺����ʵ�鷽������������ۡ����ʳɷֵ�ȷ������Ҫ����Fe��Fe3+�ļ��顢����ʽ����д��֪ʶ��

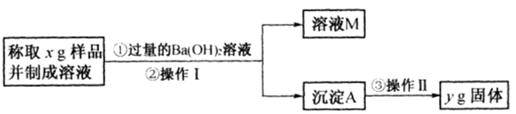
��ʵ���Ҳⶨ̼������̼�����ƵĻ�����У�̼���Ƶ���������[�÷���w(Na2CO3)��ʾ]����ȡ�˻����5.lg������ˮ�У����250mL��Һ��
a��(10��)����һ����������w(Na2CO3)���û�ѧ��Ӧ��HCO3����CO32����ȫת��Ϊ��������ȡ�������������ɴ˼���������w (Na2CO3)��
��1����ȡ100 mL���ƺõ���Һ���ձ��У��μ�����������������Һ��HCO3����CO32����ȫת��Ϊ������Ӧѡ���Լ���___________ �����ţ���
| A��CaCl2 | B��MgSO4 | C����NaCI | D��Ba(OH)2 |
��3�����ˣ���ȡ����������˲�������Ҫ�IJ���������________________________��
��4��ϴ�ӳ���������ϴ�ӳ����IJ���_____________________________��
��5�������֣���ȡ����������Ϊ9.8g���ɴ˼���w(Na2CO3)������˲��У�����δ�����־ͳ���������w (Na2CO3)________________����ƫ���ƫС����Ӱ�죩��
b�����������ζ�����w(Na2CO3)��ȡ25.00 mL���ƺõ���Һ������ƿ�У��μ�2�η�̪�Լ���ҡ�ȣ���0.2000 mol/L��������еζ����յ㡣�ظ��˲���2�Σ�������������ƽ��ֵΪ20.00 mL�� [��֪���͵�̼����ҺPHΪ3.9]
��1����ȡ25.00 mL���ƺõ���Һ��Ӧѡ��_______________��������ɡ�
��2���жϵζ��յ��������_____________________���˹����з�Ӧ�����ӷ���ʽΪ__________________________________________________��
��3���˷����w(Na2CO3)=________%��������λС����
������������������ͭ���Ǻ�ɫ��ĩ�����������ϡ�ijУһ��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ��Fe2O3��Cu2O����ߵĻ���̽���������£�
�������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4���ڿ����м�������CuO��
�������
����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ��
ȡ������ĩ��������ϡ�����У���������Һ���ٵμ�KSCN�Լ���
��1��������1��������ʵ��������_____________________________________________��
��2�����μ�KSCN�Լ�����Һ�����ɫ����֤��ԭ�����ĩ��һ����������������������Ϊ����˵��������________�������������(����д����Ӧ����ʽ)____________
________________________________________________________________________��
��3���������ĩ��ȫ�ܽ�������ڣ��μ�KSCN�Լ�ʱ��Һ�����ɫ����֤��ԭ�����ĩ��________��д��������Ӧ�����ӷ���ʽ________________________________��
̽������
��ʵ�������ȷ����ɫ��ĩΪFe2O3��Cu2O�Ļ���
��4��ʵ��С�����ü��ȷ��ⶨCu2O������������ȡa g�����ĩ�ڿ����г�ּ��ȣ����������ٱ仯ʱ����������Ϊb g(b��a)����������Cu2O����������Ϊ________��
��5��ʵ��С�������øú�ɫ��ĩ��ȡ�ϴ����ĵ���(CuSO4��5H2O)�����������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2����Fe2����Fe3���ֱ����ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
ʵ�����������Լ��ɹ�ѡ��A.��ˮ��B��H2O2��C��NaOH��D��Cu2(OH)2CO3
ʵ��С���������ʵ�鷽����
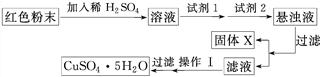
�Իش�
���Լ�1Ϊ________(����ĸ����ͬ)���Լ�2Ϊ________��
�ڹ���X�Ļ�ѧʽΪ____________________________________________________��
�۲�����Ϊ___________________________________________________________��
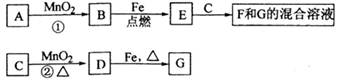

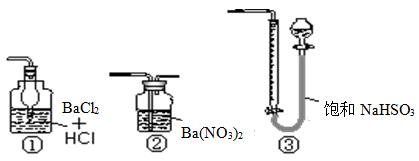
 (x��y)Cu��xCO2��(x��2y��z)H2O
(x��y)Cu��xCO2��(x��2y��z)H2O
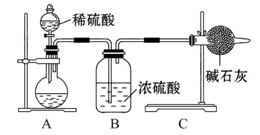

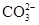 ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��