��Ŀ����
������(AlN)��һ���������ǽ������ϡ�ijAlN��Ʒ������ֺAl2O3���ʣ�Ϊ�ⶨAlN�ĺ����������������ʵ�鷽��������֪��AlN+NaOH+H2O��NaAlO2��NH3����
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��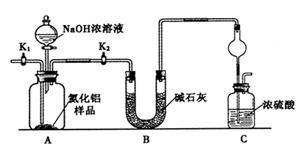
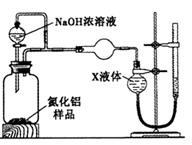
��1����ͼCװ�������θ���ܵ�������______________��
��2���������ʵ�鲽�裺��װ��ʵ��װ�ã�����____________,�ټ���ʵ��ҩƷ����������ʵ�������______________,��Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����______________��
��3������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����___________��
������2������ͼװ�òⶨm g��Ʒ��A1N�Ĵ���(���ּг�װ������ȥ)��
(
��4��Ϊ�ⶨ������������������װ���е�XҺ�������_________________________��
a��CCl4 b��H2O c��NH4Cl��Һ d��
��5����m g��Ʒ��ȫ��Ӧ�����������������ΪV mL(��ת��Ϊ��״��)����AIN����������__��
������3�������²���ⶨ��Ʒ��A1N�Ĵ��ȣ�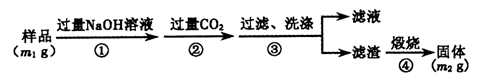
��6����������ɳ��������ӷ���ʽΪ___________________��
��7�����ڲ������δϴ�ӣ��ⶨ�����__________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
��1�������� ��2�����װ�������ԣ��ر�K1����K2����װ���в����İ���ȫ������Cװ��
��3��Cװ�ó��ڴ�����һ������װ�� ��4��ad ��5��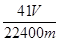
��6��AlO2����CO2��2H2O��HCO3����Al(OH)3�� ��6��ƫ��
���������������1�����ڷ�Ӧ�����ɵİ�����������ˮ��ֱ��ͨ��ˮ����������Һ�嵹����������ͼCװ�������θ���ܵ������Ƿŵ�����
��2����װ���������������������װ��ʵ��װ�ã�����Ҫ���װ�õ������ԣ�����K1��������������Խ�������ʵ������ǹر�K1����K2,��Һ©������������NaOHŨ��Һ�������ٲ������塣����װ���л������İ�������ʵ���л���Ҫͨ��Ũ�����������������������ͨ�뵪����Ŀ���ǰ�װ���в����İ���ȫ������Cװ�á�
��3������Cװ�õij���������������������е�ˮ����Ҳ�ܱ�Ũ����ϡ�ͣ��Ӷ���������˸Ľ��Ĵ�ʩ��Cװ�ó��ڴ�����һ������װ�á�
��4�����ڰ����Ǽ������壬��������ˮ����������װ���е�XҺ����������Ȼ�̼����ѡad��
��5��Vml�����ǰ������ڱ�״�������ʵ����� mol���Ľ���ԭ���غ��֪��AlN�����ʵ���Ҳ��
mol���Ľ���ԭ���غ��֪��AlN�����ʵ���Ҳ�� mol��������
mol�������� mol��41g/mol��
mol��41g/mol�� g��������������
g��������������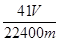 ��
��
��6����������������������Һ������ƫ�����ƣ�Ȼ��ͨ�����CO2������������������̼�����ƣ����Բ�������ɳ��������ӷ���ʽΪAlO2����CO2��2H2O��HCO3����Al(OH)3����
��7�����ڲ������δϴ�ӣ�������������ӣ���˵��������������ӣ��Ӷ����²ⶨ�����ƫ�ߡ�
���㣺�������ʴ��Ȳⶨʵ�鷽�����������

 53������ϵ�д�
53������ϵ�д�������������������ͭ���Ǻ�ɫ��ĩ�����������ϡ�ijУһ��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ��Fe2O3��Cu2O����ߵĻ���̽���������£�
�������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4���ڿ����м�������CuO��
�������
����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ��
ȡ������ĩ��������ϡ�����У���������Һ���ٵμ�KSCN�Լ���
��1��������1��������ʵ��������_____________________________________________��
��2�����μ�KSCN�Լ�����Һ�����ɫ����֤��ԭ�����ĩ��һ����������������������Ϊ����˵��������________�������������(����д����Ӧ����ʽ)____________
________________________________________________________________________��
��3���������ĩ��ȫ�ܽ�������ڣ��μ�KSCN�Լ�ʱ��Һ�����ɫ����֤��ԭ�����ĩ��________��д��������Ӧ�����ӷ���ʽ________________________________��
̽������
��ʵ�������ȷ����ɫ��ĩΪFe2O3��Cu2O�Ļ���
��4��ʵ��С�����ü��ȷ��ⶨCu2O������������ȡa g�����ĩ�ڿ����г�ּ��ȣ����������ٱ仯ʱ����������Ϊb g(b��a)����������Cu2O����������Ϊ________��
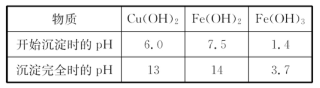
��5��ʵ��С�������øú�ɫ��ĩ��ȡ�ϴ����ĵ���(CuSO4��5H2O)�����������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2����Fe2����Fe3���ֱ����ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
ʵ�����������Լ��ɹ�ѡ��A.��ˮ��B��H2O2��C��NaOH��D��Cu2(OH)2CO3
ʵ��С���������ʵ�鷽����
�Իش�
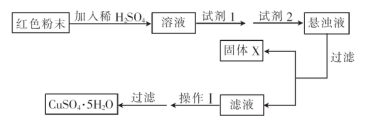
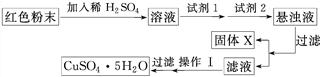
���Լ�1Ϊ________(����ĸ����ͬ)���Լ�2Ϊ________��
�ڹ���X�Ļ�ѧʽΪ____________________________________________________��
�۲�����Ϊ___________________________________________________________��
��������������Ԫ��֮һ�������Դٽ�Ѫ�쵰��������ӽ���������ҽѧ�Ͼ�����������������Ƭ����ƶѪ�IJ��˲��������������ҩƷ˵����ش����⣺
��1��ҩƬ�ϵ����¿�����__________�����ã�����ʱ�ܽ�������Ŀ����____________��
��2��ij�о�С�������KMnO4�ⶨFeSO4�ĺ�����
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250����������ʱ��Ҫ��������������ƽ���ձ�������������Ͳ�⣬����Ҫ��������__________��___________��
�ڵζ�ʱ��������������Һ����___________�����������У����������Һ����__________�����������У��ζ�ʱ����___________�����̪�����ȡ���ʯ����üӡ���ָʾ��������жϴﵽ�ζ��յ�_____________��
��3����һ�о�С������������²���������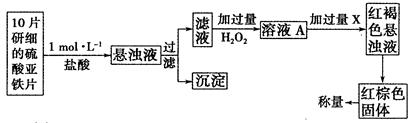
�ٴ˼�������мӹ���H2O2��Ӧ�����ӷ���ʽΪ______________��
�ڴӺ��ɫ������Һ�����ij����������������Ļ���������___________����������˳����д����
| A������ | B��ϴ�� | C����ȡ | D����Һ E����ȴ F������ |
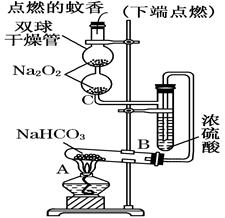
��16�֣�����ý(����ij������)�ǹ�ҵ�ϳɰ��Ĵ�����ijͬѧ������������ַ����о�����ý����ɡ�
����һ�����������̲ⶨ����ý�ĺ�������ȷ������ɡ�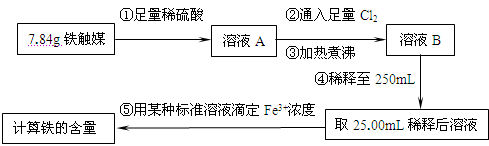
��1������ܺ������� (����������)ȡ25.00mLϡ�ͺ���Һ��
��2����Ϊͨ��Cl2������������ҺB���л����� ��Ӱ��ⶨ�����
��3����Ϊͨ��Cl2�����Ҽ�����в���֣�����ҺB���п��ܺ���Cl2�������ʵ�鷽������Cl2���������ʵ�鱨�档
��ѡ�Լ���0.1mol��L��1����KMnO4��Һ����ɫʯ����Һ��Ʒ��ϡ��Һ������-KI��Һ��0.1moL��L��1KSCN��Һ
| ʵ����� | ʵ����������� |
| | |
��������������ʵ�鷽���ⶨ����ý�ĺ�������ȷ������ɡ�
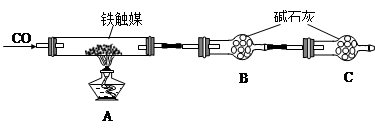
��4���������C���������� ��
��5����ȡ15.2g����ý��������ʵ�顣��ַ�Ӧ��á������B������11.0g���������ý�Ļ�ѧʽ�ɱ�ʾΪ ��(���ԭ��������C��12 O��16 Fe��56)
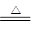 (x��y)Cu��xCO2��(x��2y��z)H2O
(x��y)Cu��xCO2��(x��2y��z)H2O


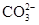 ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��