��Ŀ����
10��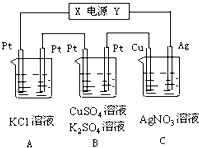 ��ͼ��ʾ�������5minʱͭ�缫����������2.16g��B��һ��Pt�缫��������0.32g���Ҵ�ʱB��������������������Իش�
��ͼ��ʾ�������5minʱͭ�缫����������2.16g��B��һ��Pt�缫��������0.32g���Ҵ�ʱB��������������������Իش���1����Դ�缫X����Ϊ������
��2��C���е�pH�仯���䣨���������С�����䡱����
��3��д��A�����������Ļ�ѧ��Ӧ����ʽ��2KCl+2H2O$\frac{\underline{\;���\;}}{\;}$2KOH+H2��+Cl2��
��4������B���в����������ڱ�״���µ��������0.224L��
���� ��1��C��ͭ�缫�������ӣ�˵��ͭ�缫Ϊ�����������缫Ϊ����������XΪ������YΪ������
��2��CΪ��Ƴأ���Һ������Ũ�Ȳ��䣻
��3��A��Ϊ���Ե缫���KCl��Һ��
��4������C�����ɵ�Ag�����ʵ������ת�Ƶĵ��ӵ����ʵ���������B������Cu����������������������ʵ��������ݵ����غ�������������������������ʵ�����Ȼ�����������������
��� �⣺��1�������5minʱͭ�缫��������2.16g��˵��ͭ�缫Ϊ�����������缫Ϊ��������XΪ������YΪ������
�ʴ�Ϊ��������
��2��CΪ��ƣ���Һ����Ũ�Ȳ��䣬��pH���䣬�ʴ�Ϊ�����䣻
��3��A���з�����ӦΪ����Ȼ�����Һ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽ2KCl+2H2O$\frac{\underline{\;���\;}}{\;}$2KOH+H2��+Cl2����
�ʴ�Ϊ��2KCl+2H2O$\frac{\underline{\;���\;}}{\;}$2KOH+H2��+Cl2����
��4����ͨ��ֱ����5minʱ��ͭ�缫��������2.16g������Ag++e-�TAg��n��Ag��=$\frac{2.16g}{108g/mol}$=0.02mol��ת�Ƶ���0.02mol��
B�еĿ�ʼһ��ʱ���ǵ������ͭ������Ϊ��4OH--4e-�TO2��+2H2O������ΪCu2++2e-�TCu��
n��Cu��=$\frac{0.32g}{64g/mol}$=0.005mol����ת�Ƶ���Ϊn��e-��=0.005mol��2=0.01mol�������ɵ�����Ϊn��O2��=0.0025mol��
����ͭ����ȫ�������Ϊ����غ����ᣬ�����ĵ缫��Ӧ������Ϊ��4OH--4e-�TO2��+2H2O������Ϊ2H++2e-�TH2����
���ݵ����غ��֪���ý�ת�Ƶĵ���Ϊn��e-����=0.02mol-0.01mol=0.01mol��
�����ɵ������������������ʵ���Ϊn�����壩=$\frac{1}{4}$��0.01+$\frac{1}{2}$��0.01=0.0075mol��
����B�е�����ɵ�����������ʵ���Ϊ0.0075mol+0.0025mol=0.01mol����V=nVm=0.01mol��22.4L/mol=0.224L��
�ʴ�Ϊ��0.224��
���� ���⿼����ԭ����������ѧ���ķ��������ͼ��������Ŀ��飬�Ѷ��еȣ�ע��������ӵķŵ�˳��Ϊ������Ĺؼ���

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�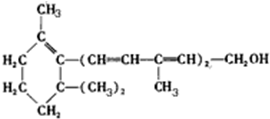
| A�� | ά����A���ڻ��� | |
| B�� | ά����A����������Ʒ�Ӧ�������� | |
| C�� | ά����A����ʹ����KMnO4��Һ��ɫ | |
| D�� | ά����A�ܷ���ȡ�����ӳɡ�������Ӧ |
| A�� | ��ϩͨ�����Ը��������Һ��ɫ | B�� | Ũ����ε�Ƥ����� | ||
| C�� | ����ƾ���������� | D�� | ��������ˮ����ˮ��ӽ���ɫ |
| A�� | ��� | B�� | ��Ӿ | C�� | ��������� | D�� | �������۲� |
| A�� | ���ԣ�HClO4��HBrO4��HIO4 | B�� | �е㣺H2O��HF��HCl��HBr | ||
| C�� | �ǽ����ԣ�F��O��S | D�� | �ȶ��ԣ�PH3��H2S��HCl |
| A�� | 3��1 | B�� | 1��3 | C�� | 3��2 | D�� | 4��1 |
| A�� | ��Ӧ��ϵ����ѹ�㶨 | B�� | A��B��C��D�����ʵ���֮��Ϊ1��3��2��2 | ||
| C�� | c��A����c��B��=1��3 | D�� | 2V��B����=3V��C���� |