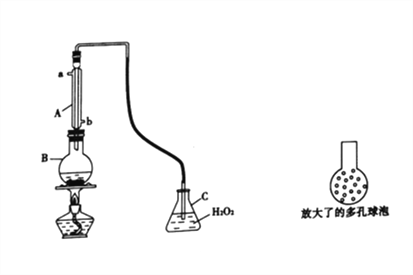
ЬтФПФкШн
ЁОЬтФПЁПаТаЭПЙРЃбёвЉШ№АЭЦЅЬиЃЌПЩБЃЛЄЮИГІ№ЄФЄУтЪмИїжжжТРЃбёвђзгЕФЮЃКІЃЌЦфКЯГЩТЗЯпШчЯТЃК
(1)AЕФЛЏбЇУћГЦЮЊ______________ЃЌCЕФКЫДХЙВеёЧтЦзОпга_________________зщЗх
(2)AгызуСПЕФNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ______________
(3)ЛЏКЯЮяFжаКЌбѕЙйФмЭХЕФУћГЦЮЊ__________________ЃЌЛЏКЯЮяFЕФЗжзгЪНЮЊ_____________
(4)ЗДгІЂй~ЂлжаЃЌЪєгкШЁДњЗДгІЕФЪЧ__________________(ЬюађКХ)
(5)CЁњDЕФзЊЛЏжаЃЌЩњГЩЕФСэвЛжжВњЮяЮЊHClЃЌдђCЁњDЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________
(6)вбжЊYжаЕФфхдзгБЛ--OHШЁДњЕУЕНZЃЌаДГіЭЌЪБТњзуЯТСаЬѕМўЕФZЕФвЛжжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК___________________
IЃЎЗжзгжаКЌгавЛИіБНЛЗКЭвЛИіЮхдЊЛЗЃЌЧвЖМЪЧЬМдзгЛЗ
IIЃЎБНЛЗЩЯгаСНИіШЁДњЛљЃЌЧвДІгкЖдЮЛ
III.ФмгыNaHCO3ШмвКЗЂЩњЗДгІ
(7)вбжЊCH3CH2OH![]() CH3CH2BrЃЌвдAКЭHOCH2CH2CH2OHЮЊдСЯжЦБИ
CH3CH2BrЃЌвдAКЭHOCH2CH2CH2OHЮЊдСЯжЦБИ![]() ЕФКЯГЩТЗЯпСїГЬЭМШчЯТЃКHOCH2CH2CH2OH
ЕФКЯГЩТЗЯпСїГЬЭМШчЯТЃКHOCH2CH2CH2OH![]() ЮяжЪX
ЮяжЪX![]() ЮяжЪY
ЮяжЪY![]()
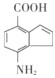
![]() ЃЌдђЮяжЪXЮЊ__________ЃЌЮяжЪYЮЊ____________
ЃЌдђЮяжЪXЮЊ__________ЃЌЮяжЪYЮЊ____________
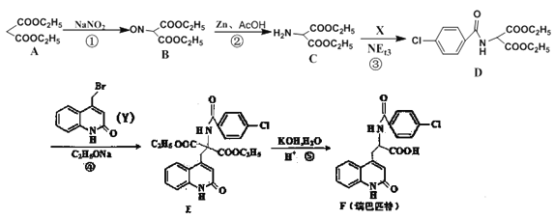
ЁОД№АИЁПБћЖўЫсЖўввѕЅ 4 C2H5OOCCH2COOC2H5ЃЋ2NaOH ![]() NaOOCCH2COONaЃЋ2C2H5OH ыФМќЁЂєШЛљ C19H15O4N2Cl ЂйЂл
NaOOCCH2COONaЃЋ2C2H5OH ыФМќЁЂєШЛљ C19H15O4N2Cl ЂйЂл 
 BrCH2CH2CH2Br
BrCH2CH2CH2Br 
ЁОНтЮіЁП
ЃЈ1ЃЉИљОнЯЕЭГУќУћЗЈЃЌгаЛњЮя ЕФЛЏбЇУћГЦЮЊБћЖўЫсЖўввѕЅЃЌCЃЈ
ЕФЛЏбЇУћГЦЮЊБћЖўЫсЖўввѕЅЃЌCЃЈ ЃЉжага4жжЧтдзгЃЌКЫДХЙВеёЧтЦзОпга4зщЗхЃЌ
ЃЉжага4жжЧтдзгЃЌКЫДХЙВеёЧтЦзОпга4зщЗхЃЌ
ЙЪД№АИЮЊЃКБћЖўЫсЖўввѕЅЃЌ4ЁЃ
ЃЈ2ЃЉѕЅПЩдкNaOHШмвКжаЗЂЩњМюадЫЎНтЃЌБћЖўЫсЖўввѕЅгызуСПNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ![]() +2NaOH
+2NaOH![]() NaOOCCH2COONa+2C2H5OHЃЌЙЪД№АИЮЊЃК
NaOOCCH2COONa+2C2H5OHЃЌЙЪД№АИЮЊЃК ![]() +2NaOH
+2NaOH![]() NaOOCCH2COONa+2C2H5OHЁЃ
NaOOCCH2COONa+2C2H5OHЁЃ
ЃЈ3ЃЉЛЏКЯЮяFЃЈ ЃЉжаЙйФмЭХЕФУћГЦЮЊыФМќЁЂєШЛљЁЂТШдзгЁЂЬМЬМЫЋМќЃЌЦфжаКЌбѕЙйФмЭХЕФУћГЦЮЊыФМќЁЂєШЛљЃЌЛЏКЯЮяFЕФЗжзгЪНЮЊC19H15O4N2ClЃЌЙЪД№АИЮЊЃКыФМќЁЂєШЛљЃЌC19H15O4N2ClЁЃ
ЃЉжаЙйФмЭХЕФУћГЦЮЊыФМќЁЂєШЛљЁЂТШдзгЁЂЬМЬМЫЋМќЃЌЦфжаКЌбѕЙйФмЭХЕФУћГЦЮЊыФМќЁЂєШЛљЃЌЛЏКЯЮяFЕФЗжзгЪНЮЊC19H15O4N2ClЃЌЙЪД№АИЮЊЃКыФМќЁЂєШЛљЃЌC19H15O4N2ClЁЃ
ЃЈ4ЃЉЗДгІЂйгЩ ЩњГЩ
ЩњГЩ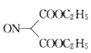 ЕФЗДгІРраЭЮЊШЁДњЗДгІЃЛЗДгІЂкЪЧ
ЕФЗДгІРраЭЮЊШЁДњЗДгІЃЛЗДгІЂкЪЧ МгЧтШЅбѕЛЙдЩњГЩ
МгЧтШЅбѕЛЙдЩњГЩ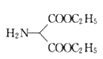 ЃЌЦфЗДгІРраЭЮЊЛЙдЗДгІЃЛЗДгІЂлЪЧ
ЃЌЦфЗДгІРраЭЮЊЛЙдЗДгІЃЛЗДгІЂлЪЧ ЕФАБЛљжаЕФ1ИіЧтдзгБЛ
ЕФАБЛљжаЕФ1ИіЧтдзгБЛ![]() ШЁДњЃЌЪЧШЁДњЗДгІЃЌЫљвдЗДгІЂй~ЂлжаЃЌЪєгкШЁДњЗДгІЕФЪЧЂйЂлЃЌЙЪД№АИЮЊЃКЂйЂлЁЃ
ШЁДњЃЌЪЧШЁДњЗДгІЃЌЫљвдЗДгІЂй~ЂлжаЃЌЪєгкШЁДњЗДгІЕФЪЧЂйЂлЃЌЙЪД№АИЮЊЃКЂйЂлЁЃ
ЃЈ5ЃЉЖдБШCЁЂDЕФНсЙЙПЩжЊЃЌCгыXЗЂЩњШЁДњЗДгІЩњГЩDКЭHClЃЌИљОнВњЮяЕФЬММмНсЙЙВЂНсКЯдзгЪиКуЃЌПЩжЊCЁњDЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК +
+![]()
![]()
 +HClЃЌ
+HClЃЌ
ЙЪД№АИЮЊЃК +
+![]()
![]()
 +HClЁЃ
+HClЁЃ
ЃЈ6ЃЉYЕФНсЙЙМђЪНЮЊЃК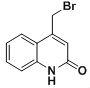 ЃЌYжаЕФфхдзгБЛ-OHШЁДњЕУЕНZЃЌдђZЕФНсЙЙМђЪНЮЊЃК
ЃЌYжаЕФфхдзгБЛ-OHШЁДњЕУЕНZЃЌдђZЕФНсЙЙМђЪНЮЊЃК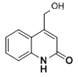 ЃЌZЕФЭЌЗжвьЙЙЬхIЃЎЗжзгжаКЌгавЛИіБНЛЗКЭвЛИіЮхдЊЛЗЃЌЧвЖМЪЧЬМдзгЛЗЃЌЫЕУїNдзгВЛдкЛЗЩЯЃЛIII.ФмгыNaHCO3ШмвКЗЂЩњЗДгІЃЌЫЕУїЗжзгжаКЌгаєШЛљЃЛIIЃЎБНЛЗЩЯгаСНИіШЁДњЛљЃЌгІИУЪЧАБЛљКЭєШЛљЃЌЧвДІгкЖдЮЛЃЌЭЌЪБТњзуЯТСаЬѕМўЕФZЕФвЛжжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ
ЃЌZЕФЭЌЗжвьЙЙЬхIЃЎЗжзгжаКЌгавЛИіБНЛЗКЭвЛИіЮхдЊЛЗЃЌЧвЖМЪЧЬМдзгЛЗЃЌЫЕУїNдзгВЛдкЛЗЩЯЃЛIII.ФмгыNaHCO3ШмвКЗЂЩњЗДгІЃЌЫЕУїЗжзгжаКЌгаєШЛљЃЛIIЃЎБНЛЗЩЯгаСНИіШЁДњЛљЃЌгІИУЪЧАБЛљКЭєШЛљЃЌЧвДІгкЖдЮЛЃЌЭЌЪБТњзуЯТСаЬѕМўЕФZЕФвЛжжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК ЁЃ
ЁЃ
(7)вбжЊCH3CH2OH![]() CH3CH2BrЃЌвдAКЭHOCH2CH2CH2OHЮЊдСЯжЦБИ
CH3CH2BrЃЌвдAКЭHOCH2CH2CH2OHЮЊдСЯжЦБИ![]() ЕФКЯГЩТЗЯпСїГЬЭМШчЯТЃК
ЕФКЯГЩТЗЯпСїГЬЭМШчЯТЃК ЃЌдђЮяжЪXЮЊBrCH2CH2CH2BrЃЌЮяжЪYЮЊЃК
ЃЌдђЮяжЪXЮЊBrCH2CH2CH2BrЃЌЮяжЪYЮЊЃК ЃЌЙЪД№АИЮЊЃКBrCH2CH2CH2BrЃЌ
ЃЌЙЪД№АИЮЊЃКBrCH2CH2CH2BrЃЌ ЁЃ
ЁЃ

 ПЮЧАПЮКѓЭЌВНСЗЯАЯЕСаД№АИ
ПЮЧАПЮКѓЭЌВНСЗЯАЯЕСаД№АИ ПЮЬУаЁзївЕЯЕСаД№АИ
ПЮЬУаЁзївЕЯЕСаД№АИ ЛЦИдаЁзДдЊПкЫуЫйЫуСЗЯАВсЯЕСаД№АИ
ЛЦИдаЁзДдЊПкЫуЫйЫуСЗЯАВсЯЕСаД№АИ ГЩЙІбЕСЗМЦЛЎЯЕСаД№АИ
ГЩЙІбЕСЗМЦЛЎЯЕСаД№АИ БЖЫйбЕСЗЗЈжБЭЈжаПМПМЕуЯЕСаД№АИ
БЖЫйбЕСЗЗЈжБЭЈжаПМПМЕуЯЕСаД№АИ вЛОэИуЖЈЯЕСаД№АИ
вЛОэИуЖЈЯЕСаД№АИ УћаЃзївЕБОЯЕСаД№АИ
УћаЃзївЕБОЯЕСаД№АИ