��Ŀ����
19�����ڻ����ķ�����ᴿ�����÷����й��ˡ�������������ȡ��ϴ�������ȷֽ�ȣ����и��������з�����ᴿӦ��ȡʲô��������1������ʯ��ˮ��Һ���г�����̼��ƿ��������ù��˷�����ȥ��
��2����ȥH2�е�CO2����ϴ��������ȥ��
��3��Na2CO3�����к���������NaHCO3�����ü��ȷֽⷽ����ȥ��
��4��ʳ����Һ������NaCl���壬�ɲ�������������
���� ��1��̼��Ʋ�����ˮ��������������ˮ��
��2��������̼��NaOH��Һ��Ӧ�����������ܣ�
��3��NaHCO3���ȷֽ�����̼���ƣ�
��4��NaCl����ˮ������ʱˮ�ӷ���
��� �⣺��1��̼��Ʋ�����ˮ��������������ˮ����ѡ����˷����룬�ʴ�Ϊ�����ˣ�
��2��������̼��NaOH��Һ��Ӧ�����������ܣ���ѡ��ϴ�������룬�ʴ�Ϊ��ϴ����
��3��NaHCO3���ȷֽ�����̼���ƣ���Na2CO3�����к���������NaHCO3�����ü��ȷֽⷽ����ȥ���ʴ�Ϊ�����ȷֽ⣻
��4��NaCl����ˮ������ʱˮ�ӷ�����ѡ�����������ᴿ���ʴ�Ϊ��������
���� ���⿼����������ᴿ�ķ�����ѡ��Ϊ��Ƶ���㣬�������ʵ����ʡ����ʲ��켰�������뷽��ԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
8����WΪ��Ҫԭ���Ʊ�T�IJ�����������
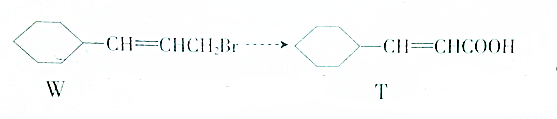
���η������л���Ӧ����Ҫ���Լ�������������������Ϊ��������
���Ȼ��⣬���� ����������ˮ��Һ������
������������ ���������ƴ���Һ�����ȣ��ữ��
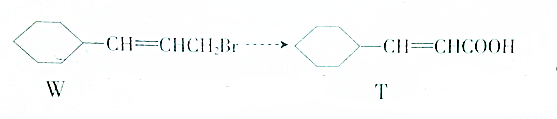
���η������л���Ӧ����Ҫ���Լ�������������������Ϊ��������
���Ȼ��⣬���� ����������ˮ��Һ������
������������ ���������ƴ���Һ�����ȣ��ữ��
| A�� | �٢ۢڢ� | B�� | �ۢ٢ܢ� | C�� | �ڢ٢ۢ� | D�� | �٢ڢۢ� |
10����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 1molNa2O2��ˮ��ȫ��Ӧʱת�Ƶ�����ΪNA | |
| B�� | 18g��ˮ��D2O�������ĵ�����Ϊ10NA | |
| C�� | 0.5molNH4HSO4�����У�����H+��ĿԼΪ0.5NA | |
| D�� | ��״��ʱ��1LpH=13��NaOH��Һ�к��е�OH-������Ϊ0.1NA |
14����ȥ����HNO3�е��������ᣬ�ɼ������ģ�������
| A�� | NaOH | B�� | BaCl2 | C�� | Zn | D�� | AgNO3 |
4����NAΪ�����ӵ�������ֵ������������ȷ���ǣ�������
| A�� | 1 molCl2������Fe��Ӧת�Ƶ�����һ��Ϊ3NA | |
| B�� | 1mol SiO2�����к�NA��SiO2���� | |
| C�� | 1 mol Na2O��Na2O2�����������������������3 NA | |
| D�� | ��״���£�22.4L��ˮ����NA��NH3���� |
8�����Ӿ硶��ɫ���㡷�����dz��չʾ�ˡ��㡱���������ͼ���������Ǿ��з�����ζ��Һ�壬����˵���У�����������ij�ֻ�ѧ���ʵ��ǣ�������
| A�� | �þƾ�������ȡijЩ�����е������㾫���Ƴ���ˮ | |
| B�� | ����ʱ��һЩ�Ͼƺ�ʳ�ף�ʹ�˸��� | |
| C�� | ����ˮϴ�����ȥ�������ˮЧ���� | |
| D�� | ����ˮ���в�ͬ����ζ������Ϊ���в�ͬ���� |
9���谢���ӵ���������ֵΪNA������˵����ȷ���ǣ�������
| A�� | 11.2L���������ķ�����Ϊ0.5NA | |
| B�� | 16g CH4���еĵ�����Ϊ8NA | |
| C�� | 7.8gNa2O2������CO2��Ӧ��ת�Ƶĵ�����Ϊ0.1NA | |
| D�� | 1L0.1mol•L-1��Na2S��Һ�к��е�S2-Ϊ0.1 NA |