��Ŀ����
 ��1���ж�����˵������ȷ����
��1���ж�����˵������ȷ������
��
����Ԫ��������ͬ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻
��ij�л�����ȫȼ�պ����ɶ�����̼��ˮ��˵�����л����бض�����̼���⡢��3��Ԫ�أ�
�ۼ����������Ļ�������ڹ����·�Ӧ�����ɵ���һ�ȼ�����Ȼ��⣻
����ϩ�������ӳɷ�Ӧ�IJ�����

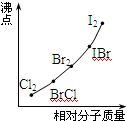
��2���Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ���ڲ�ͬ��Ӧ�жϼ�λ�ò�ͬ��
���ô�����д���пհע�������Ʒ�Ӧ��
��
��
��ѧ����ʽ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����
2CH3CH2OH+2Na��2CH3CH2ONa+H2����
����ͭ������O2��Ӧ���ٺ͢�
�ٺ͢�
��ѧ����ʽ��2CH3CH2OH+O2
2CH3CHO+2H2O
| Cu |
| �� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| Cu |
| �� |
��������1���ٸ���ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ����
�ڸ����л�����ȫȼ�պ����ɶ�����̼��ˮ��˵�����л����бض�����̼���⣬���ܺ�������
�۸��ݼ����������Ļ�������ڹ����·�Ӧ�IJ������жϣ�
�ܸ�����ϩ�������ӳɷ�Ӧ�IJ���Ϊ1��2-�������飻
��2�������Ҵ���������Ʒ�Ӧ�����Ҵ��ƺ��������Ͽ����ǻ��ϵ��������������Ҵ���ͭ������O2��Ӧ������ȩ��ˮ���Ͽ������ǻ��ϵ������������ǻ�������̼���⣮
�ڸ����л�����ȫȼ�պ����ɶ�����̼��ˮ��˵�����л����бض�����̼���⣬���ܺ�������
�۸��ݼ����������Ļ�������ڹ����·�Ӧ�IJ������жϣ�
�ܸ�����ϩ�������ӳɷ�Ӧ�IJ���Ϊ1��2-�������飻
��2�������Ҵ���������Ʒ�Ӧ�����Ҵ��ƺ��������Ͽ����ǻ��ϵ��������������Ҵ���ͭ������O2��Ӧ������ȩ��ˮ���Ͽ������ǻ��ϵ������������ǻ�������̼���⣮
����⣺��1������ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ�����ʢٴ���
�����л�����ȫȼ�պ����ɶ�����̼��ˮ��˵�����л����бض�����̼���⣬���ܺ��������ʢڴ���
��������������Ļ�������ڹ����·�Ӧ�IJ�����һ�ȼ��顢���ȼ��顢���ȼ��顢���ȼ�����Ȼ��⣬�ʢ۴���
������ϩ�������ӳɷ�Ӧ�IJ���Ϊ1��2-�������飬�ʢ���ȷ��
�ʴ�Ϊ���ܣ�
��2���Ҵ���������Ʒ�Ӧ�����Ҵ��ƺ�������2CH3CH2OH+2Na��2CH3CH2ONa+H2�����ϼ���λ��Ϊ���٣��Ҵ���ͭ������O2��Ӧ������ȩ��ˮ��2CH3CH2OH+O2
2CH3CHO+2H2O���ϼ���λ��Ϊ���ٺ͢ܣ��ʴ�Ϊ���٣�2CH3CH2OH+2Na��2CH3CH2ONa+H2�����ٺ͢ܣ�2CH3CH2OH+O2
2CH3CHO+2H2O��
�����л�����ȫȼ�պ����ɶ�����̼��ˮ��˵�����л����бض�����̼���⣬���ܺ��������ʢڴ���
��������������Ļ�������ڹ����·�Ӧ�IJ�����һ�ȼ��顢���ȼ��顢���ȼ��顢���ȼ�����Ȼ��⣬�ʢ۴���
������ϩ�������ӳɷ�Ӧ�IJ���Ϊ1��2-�������飬�ʢ���ȷ��
�ʴ�Ϊ���ܣ�
��2���Ҵ���������Ʒ�Ӧ�����Ҵ��ƺ�������2CH3CH2OH+2Na��2CH3CH2ONa+H2�����ϼ���λ��Ϊ���٣��Ҵ���ͭ������O2��Ӧ������ȩ��ˮ��2CH3CH2OH+O2
| Cu |
| �� |
| Cu |
| �� |
������������Ҫ�������Ҵ��Ļ�ѧ���ʣ����շ�Ӧ�Ļ�������д��ѧ����ʽ�ı��ϣ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
��֪25�桢101kPa�£�ʯī�ͽ��ʯȼ�յ��Ȼ�ѧ����ʽ��
C��ʯī��+O2��g��=CO2��g����H=-393.51kJ?mol��1
C�����ʯ��+O2��g��=CO2��g����H=-395.41kJ?mol��1
�ݴ��ж�����˵����ȷ���ǣ�������
C��ʯī��+O2��g��=CO2��g����H=-393.51kJ?mol��1
C�����ʯ��+O2��g��=CO2��g����H=-395.41kJ?mol��1
�ݴ��ж�����˵����ȷ���ǣ�������
| A��ʯī�Ƚ��ʯ�ȶ� | B�����ʯ��ʯī�ȶ� | C��ȼ�յ�����ʱʯī�ķų������Ƚ��ʯ�� | D��ȼ�յ�����ʱʯī�ķų���������ʯһ���� |