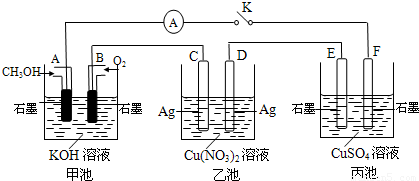
��Ŀ����
ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ�����⡣���պϸ�װ�õĵ��ʱ���۲쵽�����Ƶ�ָ�뷢����ƫת����ش��������⣺

(1)��װ�õ�������____________ (�ԭ��ء����ء�)��
(2) д���缫��Ӧʽ�� pt��______ ______��
�����в���0��1 mol����ʱ����������ͭ������ӦΪ___________________��
(3)��������Һ���䣬����缫������ͭ�缫������պ�һ��ʱ���������Һ����ɫ___________������������dz�����ޱ仯������
������Cu2+��Cl- ��Na+��SO42-���������е�����������ɵĵ������Һ�����֣���ѡ��ͭ�缫�����缫���е��ʵ�顣
(1)Ҫʹ�����������ʵ���ɺ��������䣬����ϡ��Һ��Ũ�����������ǣ�Ӧ��______Ϊ�������________��Һ�������缫��ӦʽΪ_________________________________��
(2)�Բ���������� ________ ��Һʱ����Һ�ļ�����������ǿ������Һ�����壬�����ܷ�ӦʽΪ ________________________________________��
����1��ԭ��� ��1�֣� ��2��2Cl�� - 2e���� Cl2����1�֣� 6.4g ��2�֣�
��3����ɫ���� ��2�֣�
����(1) ����1�֣� Na2SO4 ��1�֣� 4OH-��4e- = 2H2O+O2����2�֣���д2H2O-4e- = O2��+4H+ ͬ�����֣�
(2) NaCl��1�֣�
2NaCl+2H2O 2NaOH+H2��+Cl2����2�֣�
2NaOH+H2��+Cl2����2�֣�
��ûд����⡱��ͨ�硱�����֣�
����������

��1�� ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע����֪��
2Cu(s)+![]() O2(g) === Cu2O(s) ��H=��169kJ��mol-1��
O2(g) === Cu2O(s) ��H=��169kJ��mol-1��
C(s)+ ![]() O2(g) === CO(g) ��H=��110.5kJ��mol-1��
O2(g) === CO(g) ��H=��110.5kJ��mol-1��
2Cu(s)+ O2(g)=== CuO(s) ��H=��314kJ��mol-1
��ҵ����̿���ڸ��������»�ԭCuO��ȡCu2O��CO���Ȼ�ѧ����ʽΪ
��
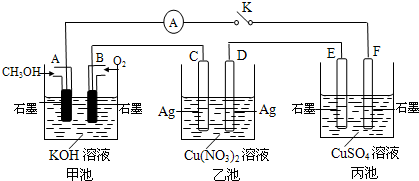
��2��ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ�����������������)�����պϸ�װ�õĵ��Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
 |
��ش��������⣺
��1���׳�Ϊ (�ԭ��ء��������ء��� ����Ƴء�)��A�缫�ĵ缫��ӦʽΪ ��
��2��������F�缫Ϊ (�����������������������������������)���óص��ܷ�Ӧ����ʽΪ
��
��3��������C����������10.8 gʱ���׳���B�缫����������O2�����Ϊ mL(��״��)��
��4��һ��ʱ��Ͽ����K������������ʹ�ҳػָ�����ӦǰŨ�ȵ��� (��ѡ����ĸ)��
A��Cu B��CuO C��Cu(OH)2 D��Cu2(OH)2CO3
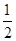 O2(g) ="==" Cu2O(s) ��H=��169kJ��mol-1��
O2(g) ="==" Cu2O(s) ��H=��169kJ��mol-1��
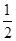 O2(g) ="==" Cu2O(s)
��H=��169kJ��mol-1��
O2(g) ="==" Cu2O(s)
��H=��169kJ��mol-1��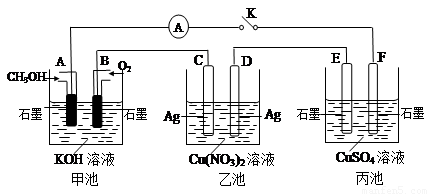
 O2��g���TCu2O��s����H=-169kJ?mol-1��
O2��g���TCu2O��s����H=-169kJ?mol-1�� O2��g���TCO��g����H=-110.5kJ?mol-1��
O2��g���TCO��g����H=-110.5kJ?mol-1��