��Ŀ����
��1������Cu2O���ھ��������Ĵ����ܶ��ܵ���ע����֪��2Cu��s��+
 O2��g���TCu2O��s����H=-169kJ?mol-1��
O2��g���TCu2O��s����H=-169kJ?mol-1��C��s��+
 O2��g���TCO��g����H=-110.5kJ?mol-1��
O2��g���TCO��g����H=-110.5kJ?mol-1��2Cu��s��+O2��g���TCuO��s����H=-314kJ?mol-1
��ҵ����̿���ڸ��������»�ԭCuO��ȡCu2O��CO���Ȼ�ѧ����ʽΪ______��
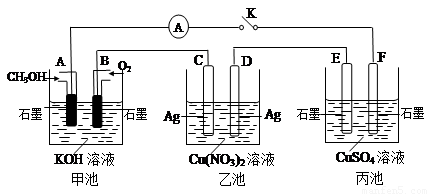
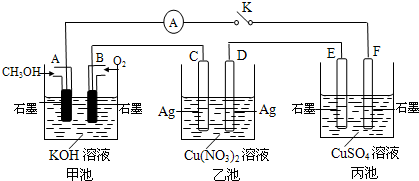
��2��ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ�����⣨�ס��ҡ������������������������պϸ�װ�õĵ��Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
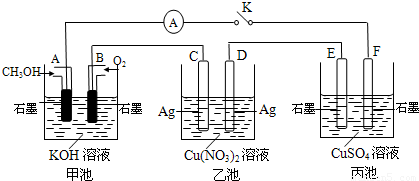
��ش��������⣺
��1���׳�Ϊ______���ԭ��ء��������ء���Ƴء�����A�缫�ĵ缫��ӦʽΪ______��
��2��������F�缫Ϊ______ ������������������������������������������óص��ܷ�Ӧ����ʽΪ______ 2H2SO4+2Cu+O2��
��2���ٸ���ȼ�ϵ�صĹ���ԭ��֪ʶ��������ȼ���Ϸ���ʧ���ӵ�������Ӧ���ش�
�ڸ��ݵ��������������жϷ����Լ����صĹ���ԭ�����ش�
�۸��ݵ����غ�������ش�
�ܸ�����Һ��ԭ�ķ�������ʲô��ʲô���ش�
����⣺��1����֪����2Cu��s��+
 O2��g���TCu2O��s����H=-169kJ?mol-1��
O2��g���TCu2O��s����H=-169kJ?mol-1����C��s��+
 O2��g���TCO��g����H=-110.5kJ?mol-1��
O2��g���TCO��g����H=-110.5kJ?mol-1����2Cu��s��+O2��g���TCuO��s����H=-314kJ?mol-1
��̿���ڸ��������»�ԭCuO��ȡCu2O��CO�Ļ�ѧ����ʽΪC��s��+2CuO ��s��=Cu��s��+CO��g����
�÷�Ӧ�����Ǣ�-��-
 ×�٣���Ӧ���ʱ���-110.5kJ?mol-1-��-314kJ?mol-1��-
×�٣���Ӧ���ʱ���-110.5kJ?mol-1-��-314kJ?mol-1��- ×��-169kJ?mol-1��=34.5kJ?mol-1��
×��-169kJ?mol-1��=34.5kJ?mol-1���ʴ�Ϊ��C��s��+2CuO ��s��=CuO��s��+CO��g����H=+34.5kJ?mol-1��
��2���ټ׳���ȼ�ϵ�أ�����ԭ��أ��ڸ�ȼ�ϵ���У��������Ǽ״�����ʧ���ӵ�������Ӧ���ڼ��Ի����£�CH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ��ԭ��أ�CH3OH+8OH--6e-=CO32-+6H2O��
�ڼ׳��У�ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫������������F��������E��������D��������C���������������ͭ��Һ�ĵ缫��ӦʽΪ��2CuSO4+2H2O
 2H2SO4+2Cu+O2�����ʴ�Ϊ��������2CuSO4+2H2O
2H2SO4+2Cu+O2�����ʴ�Ϊ��������2CuSO4+2H2O 2H2SO4+2Cu+O2����
2H2SO4+2Cu+O2������C���ϵĵ缫��ӦΪ��Ag-e-=Ag+��B�缫�ϵĵ缫��ӦΪ��O2+2H2O+4e-=4OH-��������C����������10.8gʱ����ת�Ƶ�����0.1mol����ʱ�׳���B�缫����������O2�����ʵ�����0.25mol�����Ϊ0.25mol×22.4L/mol=0.56L=560mL���ʴ�Ϊ��560��
���ҳ��ӵ������ͭ���û��õ缫������Ϊ��������������������ͭ���ӵõ������ɽ���ͭ�ķ�Ӧ����Һ�м�����CuԪ�ص�����������Ҫ�õ���ʸ�ԭ����Ҫ�������ͭ����ѡA��
������������һ���Ȼ�ѧ�͵绯ѧ��ϵ��ۺϿ����⣬Ҫ��ѧ������֪ʶ�����������ǽ���Ĺؼ����ѶȲ���

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д���1�� ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע����֪��
2Cu(s)+![]() O2(g) === Cu2O(s) ��H=��169kJ��mol-1��
O2(g) === Cu2O(s) ��H=��169kJ��mol-1��
C(s)+ ![]() O2(g) === CO(g) ��H=��110.5kJ��mol-1��
O2(g) === CO(g) ��H=��110.5kJ��mol-1��
2Cu(s)+ O2(g)=== CuO(s) ��H=��314kJ��mol-1
��ҵ����̿���ڸ��������»�ԭCuO��ȡCu2O��CO���Ȼ�ѧ����ʽΪ
��
��2��ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ�����������������)�����պϸ�װ�õĵ��Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
 |
��ش��������⣺
��1���׳�Ϊ (�ԭ��ء��������ء��� ����Ƴء�)��A�缫�ĵ缫��ӦʽΪ ��
��2��������F�缫Ϊ (�����������������������������������)���óص��ܷ�Ӧ����ʽΪ
��
��3��������C����������10.8 gʱ���׳���B�缫����������O2�����Ϊ mL(��״��)��
��4��һ��ʱ��Ͽ����K������������ʹ�ҳػָ�����ӦǰŨ�ȵ��� (��ѡ����ĸ)��
A��Cu B��CuO C��Cu(OH)2 D��Cu2(OH)2CO3
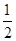 O2(g) ="==" Cu2O(s) ��H=��169kJ��mol-1��
O2(g) ="==" Cu2O(s) ��H=��169kJ��mol-1��
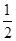 O2(g) ="==" Cu2O(s)
��H=��169kJ��mol-1��
O2(g) ="==" Cu2O(s)
��H=��169kJ��mol-1��