��Ŀ����
����Ŀ����ͨ��������,����ͼ��ʾװ�����Ҷ�ȩ(OHC-CHO)�Ʊ��Ҷ���(HOOC-COOH)���䷴ӦΪ:OHC-CHO+2Cl2+2H2O![]() HOOC-COOH+4HCl������˵����ȷ����
HOOC-COOH+4HCl������˵����ȷ����

A. Pt1�ĵ缫��ӦΪ:2H2O+ 2e-=2OH-+H2��
B. �������ṩCl- ����ǿ�����Ե�����
C. ÿ����0.1mol�Ҷ�ȩ����Pt1���ų�2.24 L ����(��״��)
D. ÿ�õ�1mol�Ҷ��Ὣ��2 mol H+������Ǩ�Ƶ�����
���𰸡�B
��������A��Pt1�缫Ϊ������H+�õ���������������2H++2e-=H2����Aѡ�������ɵ�OH-�������Ṳ�棬A����B��������Cl-ʧȥ��������Cl2�����Ҷ�ȩ����Ϊ�Ҷ��ᣬͬʱ������ǿ��Һ�ĵ����ԣ�B��ȷ��C������0.1mol�Ҷ�ȩʱ��������0.2molCl2��ת�Ƶ�����ĿΪ0.4mol��������������H2�����ʵ���Ϊ0.2mol���ڱ�״���µ����Ϊ4.48L��C����D��ÿ�õ�1mol�Ҷ��ᣬ������2molCl2��ͬʱ����4molHCl�������ϱض�����4molCl-�������ϵ���4molH+��Ϊ�˱��ֵ����ԣ����ӽ���ĤӦʹ�������ӽ���Ĥ�����Ҳ�����4molH+��Ǩ�Ƶ����ң�D������ȷ��ΪB��

 ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�����Ŀ��ʯ�Ͳ�Ʒ�г�����H2S�⣬�����и�����̬���л�����COS��CH3SH�ȡ�
�ش��������⣺
��1��CH3SH�������ĵ���ʽΪ__________��
��2��CO��H2S��Ӧ�ɲ����ʻ���( COS)����һ�����ܱ������з�����Ӧ��CO(g)+H2S(g)![]() COS(g)+H2(g)���ﵽƽ�⣬�������±���ʾ��
COS(g)+H2(g)���ﵽƽ�⣬�������±���ʾ��
ʵ�� | �¶�/�� | ��ʼʱ | ƽ��ʱ | |||
n(CO)/mol | n(H2S)/mol | n(COS)/mol | n(H2)/mol | n(CO)/mol | ||
1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
�ٸ÷�Ӧ��________��Ӧ������ȡ����ȡ�����
��ʵ��1��ƽ��ʱ��CO��ת����Ϊ_______��
��ʵ��2�ﵽƽ��ʱ��a_______7.0�����������С�ڡ����ڡ�����
��ʵ��3��ƽ����ٳ���1.0 molH2��ƽ�ⳣ��ֵ____�������������С�����䡱����
��3��COS�Ǵ�����Ⱦ���������Һ�п���H2O2����COS����һ��ǿ�������ѳ���Ӧ�Ļ�ѧ����ʽΪ_______________��
��4����һ��������Ϊ�����K2CO3һ����˹����
��K2CO3��Һ����H2S�ķ�ӦΪK2CO3 +H2S =KHS +KHCO3���÷�Ӧ��ƽ�ⳣ���Ķ���ֵΪlgK=_____����֪��H2CO3 lgK1=-6.4��lgK,2=- 10.3��H2S lgKl=-7.0��lgK
����֪�����Ȼ�ѧ����ʽ��
a. 2H2S(g)+3O2(g)=2SO2(g)+2H2O(1) ��H1=-1172kJ/mol
b. 2H2S(g)+O2(g)=2S(s)+2H2O(1) ��H2 = 632 kJ/mol
����˹��������ķ�ӦΪSO2��H2S���巴Ӧ����S(s)����÷�Ӧ���Ȼ�ѧ����ʽΪ_________��
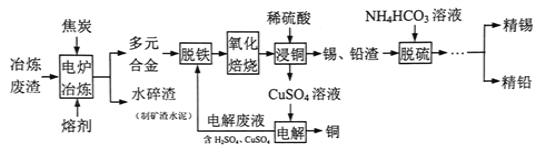
����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO(MgOռ40%,����CaO��MnO��Fe2O3��FeO��Al2O3��SiO2������)���Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO4��7H2O����������:
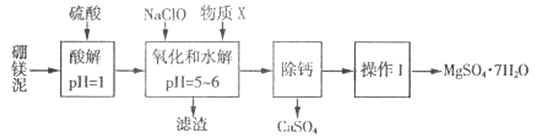
��������ش���������:
(1)ʵ��������1mol/L������800 mL������18.4 mol/L��Ũ���������ƣ�����ȡŨ����ʱ����ʹ�õ���Ͳ���Ϊ_______��(����ĸ)
A.10 mL B.25 mL C.50 mL D.100 mL
(2)�����NaClO �������Һ��Mn2+ ��Ӧ:Mn2+ + ClO-+H2O= MnO2 ��+ 2H+ + Cl-����Һ�л���һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ___________���ò�����,����X��________(�ѧʽ)��
(3)���������г�MnO2��CaSO4 ���_______��(�ѧʽ)
(4)��֪MgSO4��CaSO4���ܽ�����±�:
�¶�/�� | 40 | 50 | 60 | 70 | |
�ܽ��/g | MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 | |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ,�����ϱ�����,��Ҫ˵����������:____________��
(5)������I���ǽ���Һ����һϵ�в�������ո�����á���ո����ԭ����________��
(6)ʵ�����ṩ����þ�100g���õ���MgSO4��7H2O Ϊ147.6 g����MgSO4��7H2O�IJ���Ϊ________%��