��Ŀ����
����Ŀ��ijǿ������ɫ��Һ�п��ܺ��±��е����������ӡ�
������ | Mg2+��NH4+��Ba2+��Al3+��Fe2+ |
������ | SiO32-��MnO4����Cl����NO3����SO32���� |
ʵ���ȡ��������Һ��������ʵ�顣
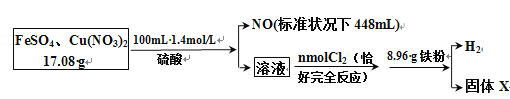
ʵ���Ϊ�˽�һ��ȷ������Һ����ɣ�ȡ100mLԭ��Һ�������Һ�еμ�1mol��L-1��NaOH��Һ����������������������������Һ����Ĺ�ϵ��ͼ��ʾ��

�ش��������⣺
��1��������ʵ��Ϳ����ƶϳ����ϱ��е�����һ�������ڵ���____________�֡�
��2��ͨ��ʵ������ȷ������Һ��һ�����ڵ���������________________��
��3��д��ʵ����ͼ����BC�ζ�Ӧ�����ӷ���ʽ��_______________________________________________________________��
��4��A���Ӧ�Ĺ�������Ϊ____________g��
��5������Һ�������ӵ�Ũ��Ϊ____________mol��L��1
���𰸡�4NO3��Al(OH)3��OH��=Al[(OH)4]����Al(OH)3��OH��=AlO2����2H2O0.1360.08
��������
ijǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����������һ������Cl-��������������������һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��Ϊ�˽�һ��ȷ������Һ����ɣ�ȡ100mLԭ��Һ�������Һ�еμ�1molL-1��NaOH��Һ�����ݲ�������������������������Һ����Ĺ�ϵ���õ�һ������Al3+�����ݴ��ڵ������Լ�������������ݵ���غ�ȷ�����������Ƿ���ڣ��ݴ˽��
��1��ijǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���һ�������ڵ���4�֣�
��2��ǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����������һ������Cl-��������������������һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��������Һ�Ե����Կ�֪����Һ��һ�����ڵ���������NO3-��
��3��ʵ����ͼ����BC�����������������������ƵĹ��̣���Ӧ�����ӷ���ʽΪAl(OH)3��OH��=Al[(OH)4]����Al(OH)3��OH��=AlO2����2H2O��
��4��BC�ζ�Ӧ�����ӷ���ʽAl(OH)3��OH��=AlO2����2H2O�����ĵ�����������0.001mol�����Ժ�����������0.001mol������þ���Ӻ�������һ��������������0.005mol������þ�������ʵ���Ҳ��0.001mol��A��õ��Ĺ�����������þ0.001mol����������0.001mol��������0.001mol��58g/mol+0.001mol��78g/mol=0.136g��
��5������Һ�д��ڵ���������NO3-������ͼ��ʼ�����������к������ӣ�����Һ������������0.001mol��AB������������笠���Ӧ���������������笠�������0.002mol�������ӡ�þ���Ӹ���0.001mol�����ݵ���غ㣬������������ʵ���Ϊ0.001mol+0.002mol+0.002mol+0.003mol��0.008mol��Ũ����0.008mol��0.1L��0.08mol/L��

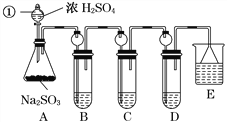
����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ�������У�Ԥ���������ʵ���������

ѡ�� | �������� | �������� | Ԥ�����е����� |
A�� | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B�� | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C�� | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D�� | ������Һ | �������������Һ | ��Һ����ɫ |
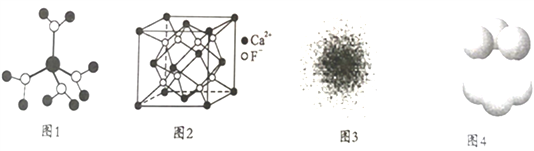
����Ŀ�������ģ����и�ѡ���йض�Ӧ�л����˵����ȷ����(����)
A |
| �����18��ԭ�Ӵ���ͬһƽ���� |
B |
| �����ϵ�̼ԭ������5 |
C |
| �������Եõ�3�������� |
D |
| ��������1��3�� 4�������� |
A. A B. B C. C D. D