ћвƒњƒЏ»Ё
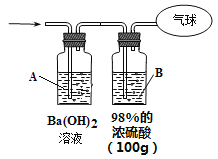
°Њћвƒњ°њ°ЊЋЂЅч÷–—І2017љмѕ¬—І∆ЏµЏґюіќ ”¶–‘њЉ ‘°њƒ≥ѕыґЊ“Їµƒ÷ч“™≥…Ј÷ќ™NaClЇЌNaClO£ђ‘Џњ’∆ш÷–“„ќь ’CO2ґш±д÷ £ђ«“NaClЇЌNaClO‘ЏЋб–‘ћхЉюѕ¬њ…ЈҐ…ъЈі”¶£ЇClO- + Cl- + 2H+ = Cl2°ь+ H2O°£ƒ≥—Іѕ∞–°„йƒвћљЊњЄ√ѕыґЊ“Їµƒ±д÷ «йњц°£
£®1£©»° ЅњѕыґЊ“ЇЈ≈‘Џ ‘є№÷–£ђЉ”»л„гЅњ“їґ®≈®ґ»µƒЅтЋб£ђ”–∆шћеЈ≈≥ц°£Ќ®єэѕ¬Ѕ–„∞÷√Љм—й∆шћеµƒ≥…Ј÷њ…“‘≈–ґѕѕыґЊ“Ї «Јс±д÷ °£

—Іѕ∞–°„й—–ЊњЇу»ѕќ™±д÷ «йњцњ…ƒ№”–»э÷÷£ЇЉ„£Ї≤њЈ÷±д÷ £ї““£Їќі±д÷ £ї±ы£Ї______°£
ќ™ЅЋ—й÷§њ…ƒ№ќ™Љ„£ђ«лЌк≥…ѕ¬Ѕ– µ—йЈљ∞Є°£ѕё—° ‘ЉЅ£Ї
A£Ѓ98%µƒ≈®ЅтЋб B£Ѓ1%µƒ∆ЈЇм»№“Ї C£Ѓ1.0 mol°§L-1µƒKI-µнЈџ»№“Ї
D£Ѓ1.0 mol°§L-1 µƒNaOH»№“Ї e.≥ќ«е ѓї“ЋЃ f.±•ЇЌNaCl»№“Ї
ЋщЉ” ‘ЉЅ | ‘§∆Џѕ÷ѕуЇЌљб¬џ |
‘є№A÷–Љ”„гЅњ______(ћо–тЇ≈£©£ї ‘є№B÷–Љ”1%∆ЈЇм»№“Ї£ї ‘є№C÷–Љ”______(ћо–тЇ≈)°£ | »фA÷–________£ђ B÷–________£ђ C÷–________£ђ‘тЉ„≥…ЅҐ°£ |
£®2£©”√µќґ®Ј®≤вґ®ѕыґЊ“Ї÷–NaClOµƒ≈®ґ»°£
Ґў‘Џ є”√µќґ®є№÷Ѓ«∞ „ѕ»љш––µƒ≤ў„ч «_____________________£ї
ҐЏЅњ»°25.00 mLѕыґЊ“ЇЈ≈»л„ґ–ќ∆њ÷–£ђЉ”»лєэЅњµƒa mol°§L-1 Na2SO3»№“Їv1 mL£ї£®Јі”¶µƒїѓ—ІЈљ≥ћ љќ™£ЇNaClO + Na2SO3 = NaCl+ Na2SO4£©љЂb mol°§L-1µƒ”√ЅтЋбЋбїѓµƒKMnO4»№“Ї„∞»л_________(ћо“«∆ч√ы≥∆)÷–£їµќґ® £”аµƒNa2SO3»№“Ї£ђЈі”¶µƒїѓ—ІЈљ≥ћ љќ™£Ї_______________°£µ±»№“Ї”…__________(ћоµќґ®÷’µгѕ÷ѕу)Ќ£÷єµќґ®£ђЉ«¬Љ эЊЁ°£
Ґџ÷ЎЄі…ѕ цµќґ®≤ў„ч2іќ£ђ∆љЊщѕыЇƒЋб–‘KMnO4»№“Їv2 mL°£‘тѕыґЊ“Ї÷–NaClOµƒ≈®ґ»___mol°§L-1(”√Їђa°Ґb°Ґv1°Ґv2µƒіъ э љ±н Њ)°£
°Њір∞Є°њ»Ђ≤њ±д÷ c A÷–»№“Ї±дјґ…Ђ B÷–»№“Ї≤їЌ …Ђ e C÷–»№“Ї±дїл„« Љм≤йµќґ®є№ «Јс¬©“Ї£®їтЉм¬©£© Ћб љµќґ®є№
2KMnO4+5Na2SO3+3H2SO4£љK2SO4+2MnSO4+5Na2SO4+3H2O ”…ќё…Ђ±дќ™«≥„ѕ…Ђ£ђ«“∞лЈ÷÷”≤їЌ …Ђ (2av1-5bv2)/50
°Њљвќц°њ£®1£©ЄщЊЁћв“в£ђ±д÷ «йњцњ…ƒ№”–»э÷÷£ЇЉ„£Ї≤њЈ÷±д÷ £ї““£Їќі±д÷ £ї±ы£Ї»Ђ≤њ±д÷ £ї“™Ћµ√ч≤њЈ÷±д÷ £ђ‘т–и“™Љм—й≤ъ…ъµƒ∆шће”–¬»∆шЇЌґю—хїѓћЉ£ђ“™ѕ»Њя”–¬»∆ш£ђ≤Ґ«“≥э»•¬»∆ш£ђЋщ“‘A÷–Љ”»л„гЅњµƒ1.0 mol°§L-1µƒKI-µнЈџ»№“Ї£ђЉм—й≤Ґ≥э»•¬»∆ш£їB÷–Љ”»л∆ЈЇм»№“ЇЉм—鬻∆ш «Јс≥эЊ°£ђC„∞÷√÷–Љ”»л≥ќ«е ѓї“ЋЃЉм—йґю—хїѓћЉ£ђє ір∞Єќ™£Ї»Ђ≤њ±д÷ £ї c£їA÷–»№“Ї±дјґ…Ђ£їB÷–»№“Ї≤їЌ …Ђ£їe£їC÷–»№“Ї±дїл„«
£®2£©Ґў‘Џ є”√µќґ®є№÷Ѓ«∞“™≤鬩£ђє ір∞Єќ™£ЇЉм≤йµќґ®є№ «Јс¬©“Ї£ї
ҐЏ”√ЅтЋбЋбїѓµƒKMnO4»№“ЇЊя”–—хїѓ–‘£ђƒ№єїЄѓ і»йљЇє№£ђ є”√ ±÷їƒ№„∞‘ЏЋб љµќґ®є№÷–£їЄя√ћЋбЉЎ”лNa2SO3»№“Ї£ђЈі”¶µƒїѓ—ІЈљ≥ћ љќ™2KMnO4+5Na2SO3+3H2SO4=K2SO4+2MnSO4+5Na2SO4+3H2O£ђµ±»№“Ї”…ќё…Ђ±дќ™«≥„ѕ…Ђ£ђ«“∞лЈ÷÷”≤їЌ …Ђ£ђ±н ЊіпµљЅЋµќґ®÷’µг£ђє ір∞Єќ™£ЇЉм≤йµќґ®є№ «Јс¬©“Ї(їтЉм¬©)£їЋб љµќґ®є№£ї2KMnO4+5Na2SO3+3H2SO4=K2SO4+2MnSO4+5Na2SO4+3H2O £ї”…ќё…Ђ±дќ™«≥„ѕ…Ђ£ђ«“∞лЈ÷÷”≤їЌ …Ђ£ї
ҐџЄщЊЁµ√ Іµз„” ЎЇг£ђ—«ЅтЋбƒ∆ І»•µƒµз„””…Єя√ћЋбЉЎЇЌіќ¬»Ћбƒ∆Ѕљ÷÷ќп÷ µ√µљ£ђNa2SO3µƒќп÷ µƒЅњќ™v1 °Ѕ10-3L°Ѕa mol/L= a v1 °Ѕ10-3mol£ђKMnO4µƒќп÷ µƒЅњќ™v2 °Ѕ10-3L°Ѕb mol/L=b v2°Ѕ10-3mol£ђ∆д÷–KMnO4Јі”¶µƒ—«ЅтЋбƒ∆Јі”¶µƒќп÷ µƒЅњќ™![]() b v2°Ѕ10-3mol£ђ”ліќ¬»Ћбƒ∆Јі”¶µƒ—«ЅтЋбƒ∆Јі”¶µƒќп÷ µƒЅњќ™a v1 °Ѕ10-3mol -
b v2°Ѕ10-3mol£ђ”ліќ¬»Ћбƒ∆Јі”¶µƒ—«ЅтЋбƒ∆Јі”¶µƒќп÷ µƒЅњќ™a v1 °Ѕ10-3mol -![]() b v2°Ѕ10-3mol=£ђ“тіЋ(a v1 °Ѕ10-3 -
b v2°Ѕ10-3mol=£ђ“тіЋ(a v1 °Ѕ10-3 -![]() b v2°Ѕ10-3)mol£ђ‘тc(NaClO)=
b v2°Ѕ10-3)mol£ђ‘тc(NaClO)= 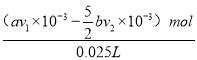 =
=![]() mol/L£ђє ір∞Єќ™£Ї
mol/L£ђє ір∞Єќ™£Ї ![]() °£
°£