��Ŀ����
����Ŀ��������泥�NH4ClO4��Ϊ��ɫ���壬���в��ȶ��ԣ���400 ��ʱ��ʼ�ֽ�����������壬��������������ƽ�����ij��ѧ��ȤС��ͬѧ��������װ�ö�NH4ClO4�ķֽ�������̽����������װ����ҩƷ�����������ּг�װ����ʡ�ԡ���
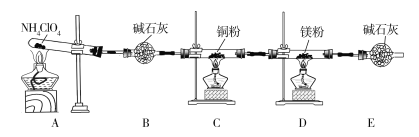
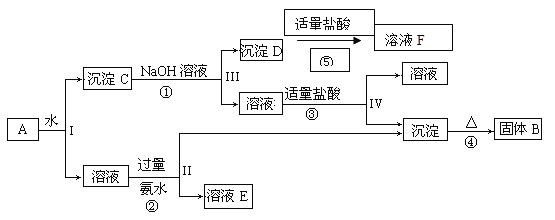
��1����ʵ������з���C��ͭ���ɺ�ɫ��Ϊ��ɫ��˵����������____________���ѧʽ�����ɡ�
��2��ʵ����Ϻ�ȡD��Ӳ�ʲ������еĹ����������Թ��У��μ�����ˮ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬����������Ļ�ѧ����ʽΪ______________��
��3��ͨ������ʵ������ķ�����ijͬѧ��Ϊ�����л�Ӧ��H2O��������Cl2����ͬѧ��Ϊ������Cl2���ڵ�������_________________________________��
��4��Ϊ��֤��H2O��Cl2�Ĵ��ڣ�ѡ����������װ�ú������ṩ��װ�ý���ʵ�飺
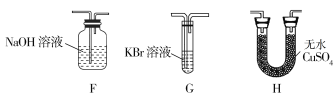
���������������ң�װ�õ�����˳��ΪA��________��________��________��
��ʵ���������G��Һ���Ϊ�Ȼ�ɫ���ñ�Ҫ�����ֺͷ���ʽ���ͳ��ָ������ԭ��________________________��
��F�з�����Ӧ�����ӷ���ʽΪ___________________________��
��5��ʵ����ۣ�NH4ClO4�ֽ�ʱ�����������������ʣ��������立ֽ�Ļ�ѧ����ʽΪ________________________________________________��
��6����ʵ�����������E��װ�м�ʯ�ҵ�Ŀ����________________��ʵ�������ijͬѧ��ͨ������D��þ�������ı仯�����������淋ķֽ��ʣ�����ɼ�����__________���ƫ��ƫС�������жϡ�����
���𰸡���1��O2
��2��Mg3N2+6H2O![]() 3Mg(OH)2��+2NH3��
3Mg(OH)2��+2NH3��
��3��O2��N2��������������ݻ��ϼ۱仯���ɣ���Ӧ���ڻ�ԭ����Ӷ��жϳ���Ԫ�صļ�̬���ͣ���������Cl2�����Ҫ�㼴�ɣ�
��4����H G F
����KBr��Һ��ͨ��Cl2��������Ӧ��2KBr+Cl2![]() 2KCl+Br2�����ɵ�������ˮʹ��Һ�ʳȻ�ɫ
2KCl+Br2�����ɵ�������ˮʹ��Һ�ʳȻ�ɫ
��Cl2+2OH![]() Cl+ClO+H2O
Cl+ClO+H2O
��5��2NH4ClO4![]() N2��+2O2��+Cl2��+4H2O
N2��+2O2��+Cl2��+4H2O
��6�����տ����е�CO2��ˮ���� ƫ��
����������1�����ȷֽ�����徭����ʯ�Ҹ������ʹͭ�ɺ�ɫ��Ϊ��ɫ��˵������������ͭ�����Բ����к���O2��
��2�����������Ϣ��֪D�й�����H2O��Ӧ����NH3��˵��������立ֽ�����л��е������ɣ�������þ��Ӧ���ɵ���þ������þ��ˮ��Ӧ����������þ�Ͱ�����Mg3N2+6H2O![]() 3Mg(OH)2��+2NH3����
3Mg(OH)2��+2NH3����
��3��������ԭ��Ӧ��Ԫ�صĻ��ϼ����ߺͽ����Ƕ���ͳһ�Ĺ�ϵ������Ӧ�ô��ڻ��ϼ۽��͵���������Կ������������ɡ�
��4������ˮ������������Ӧ������H����ˮ����ͭ����ˮ�Ĵ��ڣ�Ȼ������ͨ��KBr��Һ�У�������Ӧ��2KBr+Cl2![]() 2KCl+Br2�����ɵ��嵥������ˮ�ʳȻ�ɫ��Ϊ��ֹʣ��������Ⱦ�������������β����������������˳��Ϊ��A��H��G��F��F��������NaOH��Һ���������ӷ�ӦΪ��Cl2+2OH
2KCl+Br2�����ɵ��嵥������ˮ�ʳȻ�ɫ��Ϊ��ֹʣ��������Ⱦ�������������β����������������˳��Ϊ��A��H��G��F��F��������NaOH��Һ���������ӷ�ӦΪ��Cl2+2OH![]() Cl+ClO+H2O��
Cl+ClO+H2O��
��5����ʵ�������֪������立ֽ�IJ�����O2��Cl2��N2��H2O�����ݵ�ʧ�����������ƽ�÷���ʽ��2NH4ClO4![]() N2��+2O2��+Cl2��+4H2O��
N2��+2O2��+Cl2��+4H2O��
��6������F��װ�м�ʯ�ҵ�Ŀ�������տ����е�CO2��ˮ�������������е�N2��O2�Ի����D�У���ɲ�������ƫ���¼����������淋ķֽ���ƫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�