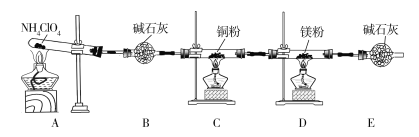
��Ŀ����
����Ŀ��ij�����A����KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
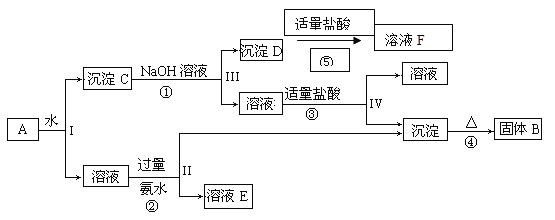
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ�����____________��
��2������������ͼ��Ӧ��ϵ��д������B��D��E�������ʵĻ�ѧʽ
����B_________________������D __________________��
��ҺE_________________________________________��
��3��д���١���������Ӧ�Ļ�ѧ����ʽ
��__________________________________����_____________________________��
��4��д���ڡ���������Ӧ�����ӷ���ʽ
��_________________________________����______________________________��
��5���ֱ�д��Al2O3��Fe2O3�ڹ�ҵ�ϵ�һ����Ҫ��;��
Al2O3__________________________Fe2O3____________________________��
���𰸡� ���� Al2O3 Fe2O3 K2SO4�ͣ�NH4��2SO4 Al2O3+2NaOH=2NaAlO2+H2O 2Al��OH��3![]() Al2O3+3H2O Al3++3NH3��H2O=Al��OH��3��+3 NH4+ AlO2-+H++H2 O=Al��OH��3�� Al2O3��ұ���� �ͻ���� Fe2O3������ԭ�� ��ɫ���� Ϳ��
Al2O3+3H2O Al3++3NH3��H2O=Al��OH��3��+3 NH4+ AlO2-+H++H2 O=Al��OH��3�� Al2O3��ұ���� �ͻ���� Fe2O3������ԭ�� ��ɫ���� Ϳ��
�������������̿�֪��Al2O3��Fe2O3������ˮ�������CΪAl2O3��Fe2O3������������Ӧ�������DΪFe2O3����Ӧ�ڢ������ɵij���ΪAl��OH��3�����ȷֽ�����BΪAl2O3����Ӧ��ΪKAl��SO4��2����ˮ�ķ�Ӧ������ҺEΪK2SO4����NH4��2SO4��NH3��H2O
��1�������IJ��ж�����Һ�ͳ����ķ��뷽��Ϊ���ˡ�
��2��������������֪��BΪAl2O3��DΪFe2O3��EΪK2SO4����NH4��2SO4��NH3��H2O��
��3����Ӧ��Ϊ��Al2O3+2OH-=2AlO2-+H2O����Ӧ��Ϊ��2Al��OH��3![]() Al2O3+3H2O
Al2O3+3H2O
��4����Ӧ��Ϊ��Al3++3NH3��H2O=Al��OH��3��+3NH4+����Ӧ��Ϊ��AlO2-+H++H2 O=Al��OH��3��
��5��Al2O3�Ǹ��۵����ʣ��ڹ�ҵ�ϵ�һ����Ҫ��;�����ͻ�����������ͻ�ܡ��ͻ�������ұ��������������Fe2O3��ҵ�ϳ��������죬�������ᡢ��ī���ȹ�ҵ�У�������������������ʯ�����������������������ԭ�ϣ�������ԭ�ϡ�