��Ŀ����
��֪��A��B��C��D��E��F����Ԫ�غ˵�����������������ڱ���ǰ�����ڵ�Ԫ�ء�����Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ�������ߣ�F���γɺ�ɫ(��ש��ɫ)��F2O�ͺ�ɫ��FO���������
�ش��������⣺
��1��F��ԭ�ӵ�M������Ų�ʽΪ ��
��2��B��C��D�ĵ�һ��������С�����˳��Ϊ ��(��Ԫ�ط��ű�ʾ)
��3��A�ļ��⻯����Ӽ�������ˮ,����Ҫԭ���� .
��4��E�������������ӵĿռ乹���� ��������ԭ�ӵ��ӻ���ʽΪ ��
��5��F�ĸ�������A�ļ��⻯���γɵ�������,��λ��Ϊ ��
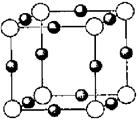
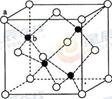
��6��A��F�γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ�����仯ѧʽΪ ������ɫ���ʾFԭ�ӣ�,��֪���ڵİ��������֮��ľ���Ϊa cm, �þ������ܶ�Ϊ g/cm3��
�ش��������⣺
��1��F��ԭ�ӵ�M������Ų�ʽΪ ��
��2��B��C��D�ĵ�һ��������С�����˳��Ϊ ��(��Ԫ�ط��ű�ʾ)
��3��A�ļ��⻯����Ӽ�������ˮ,����Ҫԭ���� .
��4��E�������������ӵĿռ乹���� ��������ԭ�ӵ��ӻ���ʽΪ ��
��5��F�ĸ�������A�ļ��⻯���γɵ�������,��λ��Ϊ ��
��6��A��F�γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ�����仯ѧʽΪ ������ɫ���ʾFԭ�ӣ�,��֪���ڵİ��������֮��ľ���Ϊa cm, �þ������ܶ�Ϊ g/cm3��

��1��3s23p63d10��2�֣�
��2��Na��Al��Si��2�֣�
��3����������ˮ����֮����������2�֣�
��4��ƽ���������Σ�2�֣� sp2��1�֣�
��5�� 4��2�֣�
��6��Cu3N ��2�֣�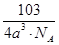 ��2�֣�������������Ҳ���֣�
��2�֣�������������Ҳ���֣�
��2��Na��Al��Si��2�֣�
��3����������ˮ����֮����������2�֣�
��4��ƽ���������Σ�2�֣� sp2��1�֣�
��5�� 4��2�֣�
��6��Cu3N ��2�֣�
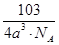 ��2�֣�������������Ҳ���֣�
��2�֣�������������Ҳ���֣����������ԭ�Ӻ���������δ�ɶԵ��ӣ�������Ų�ʽΪ1S22S22P3��ΪNԪ�أ�Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p3��ӦΪSԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�ΪAlԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬BӦΪ�ڢ�A��Ԫ�أ���ԭ��������NԪ�غ�Al֮�䣬ӦΪNaԪ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�ӦΪSiԪ�أ����ʹ�Ϊԭ�Ӿ��壬�۵��ڵ�����������ߣ�F���γɺ�ɫ(��ש��ɫ)��F2O�ͺ�ɫ��FO���������ӦΪCuԪ�أ�
��1��Cu��ԭ�ӵĵ����Ų�ʽΪ1s22s22P63s23p63d104s1��M������Ų�ʽΪ3s23p63d10
��2����Ԫ�����ڱ��У�ͬһ����Ԫ�صĵ�һ�����ܴ�����������ͬһ����Ԫ�صĵ�һ�����ܴ��ϵ�����С���ݴ˿��ж�����Ԫ�صĵ�һ�����ܵ�˳��Ϊ��Na��Al��Si
��3��A��N���ļ��⻯������ǰ�����������������ˮ,����Ҫԭ��N��O�ĵ縺��ǿ������֮���γ������
��4��S03�к���3���ļ����µ��Ӷ���Ϊ
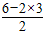 =0�����Է��ӵĿռ乹����ƽ���������Σ�sp2�ӻ�
=0�����Է��ӵĿռ乹����ƽ���������Σ�sp2�ӻ� ��5��ͭ�����백��������λ���γ������仯ѧʽΪ��[Cu(NH3)4]2+
��6�����ݾ������������ķ��䷽�����㣬�����к���ԭ�ӵ���ĿΪ8��
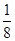 =1��ԭ�ӵ���ĿΪ��12��
=1��ԭ�ӵ���ĿΪ��12��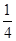 =3���ʻ�ѧʽΪCu3N�����������֮��ľ���Ϊa cm���߳�Ϊ2acm����
=3���ʻ�ѧʽΪCu3N�����������֮��ľ���Ϊa cm���߳�Ϊ2acm����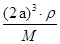 ��NA��1����æѣ�
��NA��1����æѣ�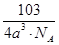 ��
��
��ϰ��ϵ�д�
 �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
 2CuCl����4H����SO42-
2CuCl����4H����SO42-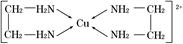

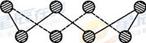
 2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��
2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��



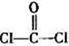 ���������______��
���������______��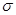 ����____��
����____��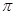 ��������Cԭ�ӵ��ӻ���ʽΪ_______��
��������Cԭ�ӵ��ӻ���ʽΪ_______��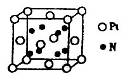 ��ʾ�������Ļ�ѧʽΪ_______��
��ʾ�������Ļ�ѧʽΪ_______��