МвДҝДЪИЭ
ЎҫМвДҝЎҝВБЎўМъФЪЙъ»оЎўЙъІъЦРУРЧЕ№г·әөДУГНҫЈ¬Зл»ШҙрПВБРОКМвЎЈ
(1) Fe2+өДЧоНвІгөзЧУЕЕІјКҪ____________ЎЈФӘЛШFeУлMnөДөЪИэөзАлДЬ·ЦұрОӘI3(Fe)ЎўI3(Mn)Ј¬ФтI3(Fe)______I3(Mn)(МоЎ°>ЎұЎўЎ°<")ЎЈ
(2)ЖшМ¬ВИ»ҜВБөД·ЦЧУЧйіЙОӘ(AlCl3)2Ј¬ЖдЦРAlЎўClҫщҙп8e-ОИ¶ЁҪб№№Ј¬AlФӯЧУөДФУ»Ҝ·ҪКҪОӘ__________ЎЈёщҫЭөИөзЧУФӯАнЈ¬AlO2-өДҝХјд№№РНОӘ_____ЎЈ
(3) Fe(CO)5өДИЫөгОӘ-20 ЎжЈ¬·РөгОӘ103 ЎжЈ¬ТЧИЬУЪТТГСЈ¬Ждҫ§МеАаРНОӘ______Ј¬
(4) ҝЖС§јТГЗ·ўПЦДіР©ә¬МъөДОпЦКҝЙҙЯ»ҜДтЛШәПіЙлВ(N2H4)Ј¬·РөгЈәN2H4>C2H6өДЦчТӘФӯТтОӘ______________________ЎЈ
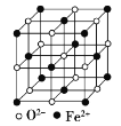
(5) FeOҫ§МеөДҫ§°ыИзНјЛщКҫЈ¬јәЦӘЈәFeOҫ§МеөДГЬ¶ИОӘҰС g/cm3Ј¬NAҙъұн°ў·ьјУөВВЮіЈКэөДЦөЎЈФЪёГҫ§°ыЦРЈ¬УлFe2+ҪфБЪЗТөИҫаАлөДFe2+КэДҝОӘ_____Ј»Fe2+УлO2-Чо¶МәЛјдҫаОӘ______pm(УГҰСәНNAұнКҫ)ЎЈ
Ўҫҙр°ёЎҝ3s23p63d6 Јј sp3 ЦұПЯРО ·ЦЧУҫ§Ме З°ХЯҝЙРОіЙ·ЦЧУјдЗвјьЈ¬әуХЯЦ»УР·¶өВ»ӘБҰ 12 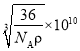
ЎҫҪвОцЎҝ
ЈЁ1Ј©әЛНвөзЧУЕЕІј°ҙДЬј¶ЛіРтЕЕІјЈ¬ФЪ»ҜС§·ҙУҰЦРК§ИҘөзЧУ°ҙҙУЧоНвІгөҪҙОНвІгТАҙОК§ИҘЈ»ёщҫЭјЫөзЧУЕЕІјКҪ·ЦОцөгөзАлДЬөДҙуРЎЈ»
ЈЁ2Ј©ёщҫЭҰТјьөзЧУ¶ФКэәН№ВөзЧУ¶ФКэЕР¶ПФУ»Ҝ·ҪКҪЈ»УГМжҙъ·Ё·ЦОцөИөзЧУМеЈ»
ЈЁ3Ј©ҪбәПҫ§МеАаРНУлРФЦКөД№ШПөЧчҙрЈ»
ЈЁ4Ј©ёщҫЭҫ§МеАаРНәН·РөгёЯөНөДұИҪПЧчҙрЈ»
ЈЁ5Ј©ёщҫЭҫ§°ыКҫТвНјҝЙЦӘЈ¬СЗМъАлЧУО»УЪҫ§°ыөД¶ҘөгәНГжРДЈ¬O2-АлЧУО»УЪҫ§°ыөДАвЙПәНМеРДЈ¬ҪбәПҰС=![]() јЖЛгЎЈ
јЖЛгЎЈ
ЈЁ1Ј©»щМ¬FeФӯЧУөДәЛНвөзЧУЕЕІјКҪОӘ[Ar]3d64s2Ј¬ФӯЧУК§ИҘөзЧУҙУЧоНвІгөҪҙОНвІгТАҙОК§ИҘЈ¬ФтFe2+өДЧоНвІгөзЧУЕЕІјКҪОӘ3s23p63d6Ј»МъФӘЛШК§ИҘөДөЪИэёцөзЧУКЗ3d6ЙПөДөзЧУЈ¬¶ш3d6ИЭТЧК§ИҘТ»ёцөзЧУРОіЙұИҪПОИ¶ЁөД3d5°лВъЧҙМ¬Ј¬¶шMnөДјЫөзЧУЕЕІјКҪОӘ3d53s2Ј¬К§ИҘөДөЪИэёцөзЧУКЗ3d5ЙПөДөзЧУЈ¬ХвКЗұИҪПОИ¶ЁөД°лідВъЧҙМ¬Ј¬ЛщТФДСК§ИҘЈ¬№КI3(Fe) ЈјI3(Mn)ЎЈ
ЈЁ2Ј©ёщҫЭЖшМ¬ВИ»ҜВБөД·ЦЧУЧйіЙОӘ(AlCl3)2Ј¬ТӘК№ГҝёцФӯЧУ¶јҙпөҪ8e-ОИ¶ЁҪб№№Ј¬AlіэБЛУлИэёцClРОіЙИэёц№ІјЫөҘјьНвЈ¬»№ТӘУЙClМṩһёцЕдО»јьЈ¬ЛщТФРиТӘІЙИЎsp3ФУ»Ҝ·ҪКҪРОіЙЛДёцөИјЫөД№ІјЫјьЈ»AlO2-ЦРә¬УР16ёцјЫөзЧУЎў3ёцФӯЧУЈ¬УлCO2Лщә¬өДФӯЧУЧЬКэЎўјЫөзЧУЧЬКэ¶јПаН¬Ј¬CO2өДҝХјд№№РНОӘЦұПЯРОЈ¬ёщҫЭөИөзЧУФӯАнЈ¬AlO2-өДҝХјд№№РНОӘЦұПЯРОЎЈ
ЈЁ3Ј©ёщҫЭFe(CO)5ИЫ·РөгөНЎўТЧИЬУЪУР»ъИЬјБөДОпАнРФЦКЈ¬ҝЙЕР¶ПЖдОӘ·ЦЧУҫ§МеЎЈ
ЈЁ4Ј©ФЪN2H4·ЦЧУЦРҙжФЪNЎӘHјьЈ¬ЛщТФДЬ№»ФЪ·ЦЧУјдРОіЙЗвјьЈ¬¶шC2H6·ЦЧУЦ»УР·¶өВ»ӘБҰЈ¬№КЗ°ХЯ·РөгёЯУЪәуХЯЎЈ
ЈЁ5Ј©УЙёГҫ§°ыҪб№№ҝЙЦӘЈ¬Fe2+ҪфБЪөДFe2+өДЧо¶МҫаАлОӘГж¶ФҪЗПЯөД![]() Ј¬ТФГжРДFe2+ОӘСРҫҝ¶ФПуЈ¬УлFe2+ҪфБЪЗТөИҫаАлөДFe2+КэДҝОӘ12ёцЈ»ёГҫ§МеЦРә¬УРөДFe2+өДёцКэОӘ
Ј¬ТФГжРДFe2+ОӘСРҫҝ¶ФПуЈ¬УлFe2+ҪфБЪЗТөИҫаАлөДFe2+КэДҝОӘ12ёцЈ»ёГҫ§МеЦРә¬УРөДFe2+өДёцКэОӘ![]() Ј¬O2-өДёцКэОӘ
Ј¬O2-өДёцКэОӘ![]() Ј¬јҙә¬УР4ёцЎ°FeOЎұЈ¬ЛщТФёГҫ§°ыөДЦКБҝОӘ
Ј¬јҙә¬УР4ёцЎ°FeOЎұЈ¬ЛщТФёГҫ§°ыөДЦКБҝОӘ![]() Ј¬Фтҫ§°ыөДұЯіӨОӘ
Ј¬Фтҫ§°ыөДұЯіӨОӘ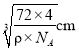 Ј¬¶шFe2+УлO2-Чо¶МәЛјдҫаОӘұЯіӨөДТ»°лЈ¬јҙ
Ј¬¶шFe2+УлO2-Чо¶МәЛјдҫаОӘұЯіӨөДТ»°лЈ¬јҙ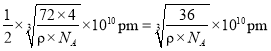 ЎЈ
ЎЈ

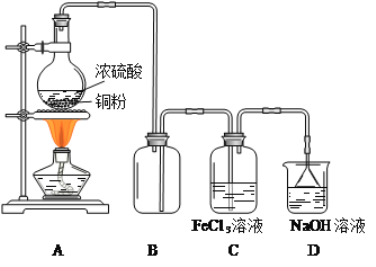
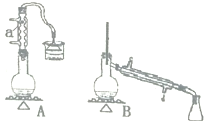
ЎҫМвДҝЎҝКөСйКТУГјУИИ1Т»¶ЎҙјЎўЕЁH2SO4әНде»ҜДЖ»мәПОпөД·Ҫ·ЁАҙЦЖұё1Т»де¶ЎНйЈ¬ЙијЖБЛИзНјЛщКҫөДКөСйЧ°ЦГ![]() ЖдЦРөДјРіЦТЗЖчТСКЎВФ
ЖдЦРөДјРіЦТЗЖчТСКЎВФ![]() ЎЈ
ЎЈ
ТСЦӘЈәH2SO4Ј«NaBr=NaHSO4Ј«HBrЈ¬ H2SO4ЈЁЕЁЈ©Ј«2HBr=Br2Ј«SO2ЎьЈ«2H2O
Зл»ШҙрПВБРОКМвЈә
(1)ТЗЖчaөДГыіЖОӘ______ЎЈ
(2)ЦЖұёІЩЧчЦРЈ¬јУИлөДЕЁБтЛбКВПИТӘҪшРРПЎКНЈ¬ЖдДҝөДКЗ______![]() МоСЎПоЧЦДё
МоСЎПоЧЦДё![]() ЎЈ
ЎЈ
![]() јхЙЩёұІъОпП©әНГСөДЙъіЙ
јхЙЩёұІъОпП©әНГСөДЙъіЙ![]() јхЙЩ
јхЙЩ![]() өДЙъіЙ
өДЙъіЙ![]() Л®КЗ·ҙУҰөДҙЯ»ҜјБ
Л®КЗ·ҙУҰөДҙЯ»ҜјБ
(3)РҙіцҙЛКөСйЦЖ1Т»де¶ЎНйөДЧЬ»ҜС§·ҪіМКҪ______ЎЈ
(4)УРН¬С§ДвНЁ№эәмНв№вЖЧТЗјш¶ЁЛщөГІъОпЦРКЗ·сә¬УРЎ°![]() ЎұЈ¬АҙИ·¶ЁёұІъОпЦРКЗ·сҙжФЪ¶ЎГС
ЎұЈ¬АҙИ·¶ЁёұІъОпЦРКЗ·сҙжФЪ¶ЎГС![]() ЗлЖАјЫёГН¬С§ЙијЖөДјш¶Ё·Ҫ°ёКЗ·сәПАнЈҝАнУЙКЗ______ЎЈ
ЗлЖАјЫёГН¬С§ЙијЖөДјш¶Ё·Ҫ°ёКЗ·сәПАнЈҝАнУЙКЗ______ЎЈ
(5)ОӘБЛҪшТ»ІҪМбҙҝ1Т»де¶ЎНйЈ¬ёГРЎЧйН¬С§ІйөГПа№ШУР»ъОпөДУР№ШКэҫЭИзұнЈә
ОпЦК | ИЫөг | ·Рөг |
1Т»¶Ўҙј |
|
|
1Т»де¶ЎНй |
|
|
¶ЎГС |
|
|
1Т»¶ЎП© |
|
|
ФтУГBЧ°ЦГНкіЙҙЛМбҙҝКөСйКұЈ»Ј¬КөСйЦРТӘСёЛЩЙэёЯОВ¶ИЦБ______КХјҜЛщөГБу·ЦЎЈ
(6)ИфКөСйЦРЛщИЎ1Т»¶ЎҙјЎўNaBr·ЦұрОӘ![]() Ўў
Ўў![]() Ј¬ЕЁБтЛб
Ј¬ЕЁБтЛб![]() Ј¬ХфіцөДҙЦІъОпҫӯПҙөУЈ¬ёЙФпәуФЩҙОХфБуөГөҪ
Ј¬ХфіцөДҙЦІъОпҫӯПҙөУЈ¬ёЙФпәуФЩҙОХфБуөГөҪ![]() Т»де¶ЎНйЈ¬Фт1Т»де¶ЎНйөДІъВККЗ______
Т»де¶ЎНйЈ¬Фт1Т»де¶ЎНйөДІъВККЗ______![]() ұЈБф2О»УРР§КэЧЦ
ұЈБф2О»УРР§КэЧЦ![]() ЎЈ
ЎЈ
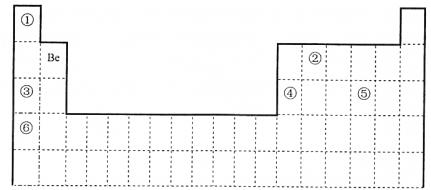
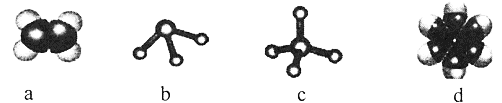
ЎҫМвДҝЎҝёщҫЭФУ»Ҝ№мөААнВЫәНјЫөзЧУ¶Ф»ҘівАнВЫДЈРНЕР¶ПЈ¬ПВБР·ЦЧУ»тАлЧУөДЦРРДФӯЧУөДФУ»Ҝ·ҪКҪј°ҝХјд№№РНХэИ·өДКЗЈЁ Ј©
СЎПо | ·ЦЧУ»тАлЧУ | ЦРРДФӯЧУФУ»Ҝ·ҪКҪ | јЫөзЧУ¶Ф»ҘівАнВЫДЈРН | ·ЦЧУ»тАлЧУөДҝХјд№№РН |
A |
|
| ЦұПЯРО | ЦұПЯРО |
B |
|
| ЖҪГжИэҪЗРО | ИэҪЗЧ¶РО |
C |
|
| ЛДГжМеРО | ЖҪГжИэҪЗРО |
D |
|
| ЛДГжМеРО | ХэЛДГжМеРО |
A.AB.BC.CD.D
ЎҫМвДҝЎҝДіСРҫҝРЎЧйҪшРРMg(OH)2іБөнИЬҪвәНЙъіЙөДКөСйМҪҫҝЎЈ
Пт2Ц§КўУР1 mL 1 molЎӨL-1өДMgCl2ИЬТәЦРёчјУИл10өО2 molЎӨL-1NaOHЈ¬ЦЖөГөИБҝMg(OH)2іБөнЈ»И»әу·ЦұрПтЖдЦРјУИлІ»Н¬КФјБЈ¬јЗВјКөСйПЦПуИзПВұнЈә
КөСйРтәЕ | јУИлКФјБ | КөСйПЦПу |
ўс | 4 mL 2 molЎӨL-1HCl ИЬТә | іБөнИЬҪв |
ўт | 4 mL 2 molЎӨL-1NH4Cl ИЬТә | іБөнИЬҪв |
ЈЁ1Ј©ҙУіБөнИЬҪвЖҪәвөДҪЗ¶ИҪвКНКөСйўсөД·ҙУҰ№эіМ_____________ЎЈ
ЈЁ2Ј©ІвөГКөСйўтЦРЛщУГNH4ClИЬТәПФЛбРФЈЁpHФјОӘ4.5Ј©Ј¬УГАлЧУ·ҪіМКҪҪвКНЖдПФЛбРФөДФӯТт___________ЎЈ
ЈЁ3Ј©јЧН¬С§ИПОӘУҰІ№ідТ»ёцКөСйЈәПтН¬СщөДMg(OH)2іБөнЦРјУ4 mLХфБуЛ®Ј¬№ЫІмөҪіБөнІ»ИЬҪвЎЈёГКөСйөДДҝөДКЗ_________ЎЈ
ЈЁ4Ј©Н¬С§ГЗІВІвКөСйўтЦРіБөнИЬҪвөДФӯТтУРБҪЦЦЈәТ»КЗNH4ClИЬТәПФЛбРФЈ¬ИЬТәЦРөДH+ҝЙТФҪбәПOH- Ј¬Ҫш¶шК№іБөнИЬҪвЈ»¶юКЗ____________ЎЈ
ЈЁ5Ј©ТТН¬С§јМРшҪшРРКөСйЈәПт4 mL 2 molЎӨL-1 NH4ClИЬТәЦРөОјУ2өОЕЁ°ұЛ®Ј¬өГөҪpHФјОӘ8өД»мәПИЬТәЈ¬ПтН¬СщөДMg(OH)2іБөнЦРјУИлёГ»мәПИЬТәЈ¬№ЫІмПЦПуЎЈ
ўЩКөСйҪб№ыЦӨГчЈЁ4Ј©ЦРөДөЪ¶юЦЦІВІвКЗіЙБўөДЈ¬ТТН¬С§»сөГөДКөСйПЦПуКЗ___________ЎЈ
ўЫТТН¬С§ХвСщЕдЦЖ»мәПИЬТәөДАнУЙКЗ___________ЎЈ
ЎҫМвДҝЎҝёЯВҜБ¶Мъ№эіМЦР·ўЙъ·ҙУҰЈә ![]() Fe2O3(s)Ј«CO(g)
Fe2O3(s)Ј«CO(g)![]()
![]() Fe(s)Ј«CO2(g)Ј¬ёГ·ҙУҰФЪІ»Н¬ОВ¶ИПВөДЖҪәвіЈКэјыУТұнЎЈПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
Fe(s)Ј«CO2(g)Ј¬ёГ·ҙУҰФЪІ»Н¬ОВ¶ИПВөДЖҪәвіЈКэјыУТұнЎЈПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
ОВ¶ИT/Ўж | 1000 | 1150 | 1300 |
ЖҪәвіЈКэK | 4.0 | 3.7 | 3.5 |
A. УЙұнЦРКэҫЭҝЙЕР¶ПёГ·ҙУҰЈә·ҙУҰОпөДЧЬДЬБҝЈјЙъіЙОпөДЧЬДЬБҝ
B. 1000ЎжПВFe2O3УлCO·ҙУҰЈ¬t minҙпөҪЖҪәвКұc(CO) =2ЎБ10-3 mol/LЈ¬ФтУГCO2ұнКҫёГ·ҙУҰөДЖҪҫщЛЩВКОӘ2ЎБ10Јӯ3/t molЎӨLЈӯ1ЎӨminЈӯ1
C. ОӘБЛК№ёГ·ҙУҰөДKФцҙуЈ¬ҝЙТФФЪЖдЛыМхјюІ»ұдКұЈ¬Фцҙуc(CO)
D. ЖдЛыМхјюІ»ұдКұЈ¬ФцјУFe2O3өДУГБҝЈ¬І»ДЬУРР§ҪөөНБ¶МъОІЖшЦРCOөДә¬Бҝ