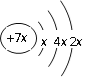��Ŀ����
����Ŀ��ij�²�ϸ�����ڵ��ⶾ�أ���ɫ��ϸ��״�ᾧ����С��������к�ǿ�Ķ��ԣ����������ѡ�Ż�¡���Ѫ�����εȣ��������������ⶾ��Ϊ��״�ģ��ṹʽ��ͼ��ʾ�����ͼ�����ش�

��1���û������к�������İ���_____________�����Ȼ�________________����
��2���û���������_____________����������ɵģ�������Щ�������������������ṹ�е�_____________��
��3����ɸû�����İ�������___________�֣�������_____________���������R����ͬ�����R����_______________��
��4���û������Ϊ��״__________�Ļ��������___________���ļ���
��5����д���߿��ڽṹ�����ƣ�A._________________��B.___________________��
��6���û��������8����ԭ�ӣ�����_____________��λ���ļ��ϣ�____________��λ��R���ϡ�
��7�����ⶾ�ػ������γɹ�����ʧȥ��______________��ˮ���ӡ�
���𰸡�(1)0 0 (2)7 R�� (3)5 3 ��CH3 (4)�� 7(5)�ļ� R�� (6)7 1 (7)7
��������
��1������İ����ṹΪ��NH2��������Ȼ��ṹΪ��COOH��
��2���û�����7���ļ�����CO��NH���������Ʋ���7����������ɣ������ͬ��R���йء�
��3������R�����Ʋⰱ������5�֣�������3����������R����ͬ�����ǣ�CH3��
��4���û�������7����������ˮ�����γɣ���Ϊ���ģ�����7���ļ���
��5��A���ļ���B��R����
��6��ÿ���ļ�����1����ԭ�ӣ�����7���ļ�����7����ԭ�ӣ�1��λ��R���ϡ�
��7����״�����γɹ����й���ȥ7��ˮ���ӡ�

����Ŀ�����÷ϱ���(��Ҫ�ɷ�ΪBaS2O3��������SiO2)Ϊԭ�������ߴ�������������������

��֪:Kap(BaS2O3)=6.96��10-11��Kap(BaF2)=1.0��10-6
(1)����ٳ�����SO2�������е���ɫ�������ɣ��÷�Ӧ�����ӷ���ʽΪ______________��
(2)����ڵ�Ŀ�����к���������������NaOH��Һ���˹�������ԭ����__________(�����ӷ���ʽ��ʾ)��
(3)��Һ����Ҫ�ɷ���BaCl2������������NaCl���ܽ���������±���
�¶� | 20�� | 40�� | 60�� | 80�� | 100�� |
NaCl | 36.0g | 36.6g | 37.3g | 39.0g | 39.8g |
BaCl2 | 35.8g | 40.8g | 46.2g | 52.5g | 59.4g |
������˲���_____ (���������ᾧ���������½ᾧ��)��
(4)��ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʡ���ת�����������뻹ԭ�������ʵ���֮��Ϊ__________��
(5)���������BaF2�ķ�Ӧ����Ϊ____________��
�����÷�Ӧ�¶ȹ��ߣ��������c(F-)���͵�ԭ����__________��
���о��������ʵ�����NH4F�ı������������BaF2�IJ��ʺʹ��ȣ���Ũ��Ϊ0. 1mol��L-1��BaCl2��Һ��0.22 mol��L-1NH4F��Һ����������������Һ��c(Ba2+)=__________ mol��L-1��
����Ŀ���±���Ԫ�����ڱ���һ���֣���ش��й����⣺
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ _________________________ ��
��2���������γ��������������Ԫ���� __________ ����Ԫ�ط��ű�ʾ����д����Ԫ���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ ________________________________ ��
��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ _________________________ ��
��4��������������������Ԫ�ص�����������ˮ������������ǿ���� ___________���ѧʽ����
��5����Ԫ�����Ԫ�����ߺ˵����֮���� ____________ ��
��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���
ʵ�鲽�� | ʵ����������� |
______________ | ________________ |