��Ŀ����
����Ŀ����1����ӦA(g)+B(g) ![]() C(g) +D(g)�����е������仯��ͼ��ʾ(E1>0��E2>0)���ش��������⡣
C(g) +D(g)�����е������仯��ͼ��ʾ(E1>0��E2>0)���ش��������⡣

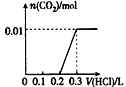
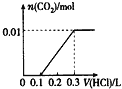
��ͼ�и÷�Ӧ��_________��Ӧ(��������������������)����Ӧ����H�ı���ʽΪ__________��
���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1_________��E2_________(����������������С������������)
��2��������һ�ּ۸�����ij���ȼ�ϣ�����Ҫ�ɷ���̼��������Ԫ�أ�ȼ�պ�ֻ�ж�����̼����̬ˮ������Ի��������Ⱦ����֪1 g������ȫȼ�շų�50.45 kJ���������Իش��������⡣������������ȼ�յ��Ȼ�ѧ����ʽΪ_______________��
��3��C3H8(g)��CH4(g)��HC��CH(g)��H2(g)��H1��+156.6kJ��mol-1��
CH3CH=CH2(g)��CH4(g)+HC��CH(g)��H2��+32.4kJ��mol-1��
����ͬ�����£���Ӧ��C3H8(g)��CH3CH=CH2(g)��H2(g)����H�� kJ��mol-1��
���𰸡�
��1������������(E2��E1)��������������
��2��C3H8(g)+5O2(g)=3CO2(g)+4H2O(L)��H=_-2219.8kJ��mol-1��
��3��+124.2��
��������
�����������1��������ͼ����Ϣ�õ�����Ӧ��������������������������Ӧ�Ƿ��ȷ�Ӧ���ʱ�=���������-��Ӧ���������ͼ�У�E1����ͨ���ӱ�Ϊ��������յ�������E2 �ǻ����֮��ķ�Ӧ���ɲ���������仯��E1-E2 �Ƿ�Ӧ��Ͳ��������֮���Ӧ�Ƿ��ȵģ������ʱ�=E1-E2 ��0���ʴ�Ϊ�����ȣ���(E2��E1)��
����������ܽ��ͷ�Ӧ�Ļ�ܣ�����E1��С��E2��С�������Է�Ӧ����������������������������Դ�С��Ӱ�죬���Բ��ı䷴Ӧ�ȵĴ�С���ʴ�Ϊ����С����С��
��2����1g������ȫȼ������Һ̬ˮʱ�ų�50.452kJ������������44g������ȫȼ������Һ̬ˮ���ų�����2219.9KJ���������ȫȼ�յ��Ȼ�ѧ����ʽ��C3H8(g)+5O2(g )��3CO2(g )+4H2O(l)��H=-2219.8KJ/mol���ʴ�Ϊ��C3H8(g)+5O2(g )��3CO2(g )+4H2O(l)��H=-2219.8 KJ/mol��
��3����C3H8(g)��CH4(g)+HC��CH(g)+H2(g)��H1=156.6kJmol-1��
��CH3CH=CH2(g)��CH4(g)+HC��CH(g)��H2=32.4kJmol-1��
���ݸ�˹������-���õ�C3H8(g)��CH3CH=CH2(g)+H2(g)��H=+124.2KJ/mol��
����ͬ�����£���ӦC3H8(g)��CH3CH=CH2(g)+H2(g)��H=+124.2KJ/mol���ʴ�Ϊ��+124.2��