��Ŀ����
����Ŀ����ȩ�ܹ��������з�Ӧ��
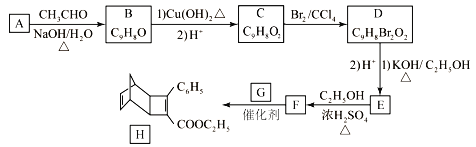
Cu2O+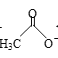
![]()
![]()

![]()

(1)Mn2���Ļ�̬�����Ų�ʽΪ____��
(2)�Ȼ�����(SOCl2)���л��ϳ�����Ҫ���Ȼ�������SOCl2��Ϊ�ȵ�����������ӵĻ�ѧʽΪ___��
(3)CH3CHO��������ԭ�ӵĹ���ӻ�������____��
(4)����ķе�(117.9 ��)����ȩ�ķе�(20.8 ��)�ߵ���Ҫԭ����____��
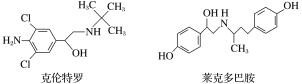
(5)�����ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ____��
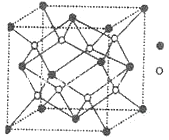
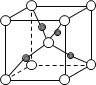
(6)��ͼ��ʾCu2O�ľ�����Cu������λ����____��
���𰸡�[Ar]3d5 SO32-��ClO3- sp2 ������Ӽ�����������ȩ���Ӽ䲻�������  ��
�� 2
2
��������
(1)���ݺ�����ӵ��Ų�����Mn2���Ļ�̬�����Ų�ʽΪ[Ar]3d5��
(2)���ݵȵ�����Ķ��壬ԭ����Ŀ��ͬ���۵�����ĿҲ��ͬ�ķ��ӣ����ӣ���Ϊ�ȵ����壬��SOCl2��Ϊ�ȵ�����������ӵĻ�ѧʽΪSO32-��ClO3-��
(3)CH3CHO��������ԭ�ӵļ۲���Ӷ�����3�����ӻ������ҲΪ3�����ӻ�������sp2��
(4)�������ȩ��Ϊ���Ӿ��壬������Ӽ�������������ȩ����֮�䲻���������������ķе�ϸߣ�
(5)��������Cu2+�ṩ�չ����4��OH���е���ԭ���ṩ�µ��Ӷԣ��γ���λ�����ʲ����ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
�� ��
��
(6)���ݾ����ṹ��ÿ��Cu����Χ����Ⱦ�������O-����Cu������λ����2��

����Ŀ���Ʊ��Ʋ�������Fe3O4����ģ��������ˮ����Ԫ�ص���Ҫʵ���������£�

��֪����CaO2��������Һ�е�FeCl2����Ӧ����Fe(OH)3��Fe3����
�ڲ��ӵ�Ca2��Ƕ��Fe3O4�У�ϴ��ʱ����ʧ������ʱ���γ�Ca3(PO4)2�ȳ�����
����Һ��pH���������������������Ӱ�졣pHԽ�ߣ��������������Խ�ࣻ pHԽ�ͣ��������������Խ�ࡣ
��1����FeCl2��FeCl3�����Һ�еμ�NaOH��Һ��һ�������·�Ӧ����Fe3O4�������ӷ���ʽΪ___________��
��2����������pH��11������������70�������½��У����˵ļ��ȷ�ʽΪ________��Ϊ��߹�����Ч�������ɲ�ȡ�Ĵ�ʩΪ_______________��
��3����Ԫ�ص�����Ч����H3PO4ˮ��Һ�к������ֲַ�������pH�Ĺ�ϵ�ֱ���ͼ1��ͼ2��ʾ��
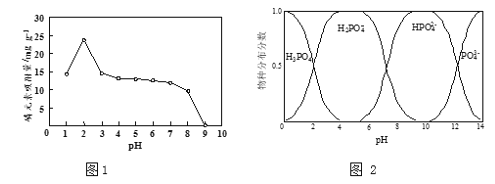
�ٲ������KH2PO4��Һģ���ˮ���������������(pH > 2)����Ԫ���������ϴ�ԭ���ǣ�pHԽ�ͣ��������������������Խ�࣬���������������ӣ�___________________
�ڲ������������ȡ�����ü�Һ���������ס���ϱ������ݣ������Ʋ�������Fe3O4������������������������ȵ������У�________��
��ͬ���������������������������Ƚ�
������ | ����Ʒ | ��Fe3O4 | �մɲ��� |
������/mg��g��1 | 24.1 | 5.0 | 12.5 |
��4������ƴӲ����Ӧ���������ƿ�л�ȡ�Ʋ�������Fe3O4��Ʒ��ʵ�鷽�����ô��������������Һ���룬______________����ɸ��ɸ�ֵõ���Ʒ (ʵ������ʹ�����Լ��������У�����ˮ����ˮ�Ҵ���pH�ơ��в�������)��
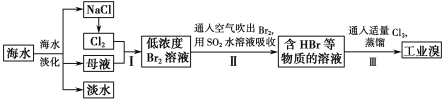
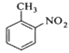
����Ŀ���������ױ���ҽҩ��Ⱦ�ϵȹ�ҵ��һ����Ҫ�л��м��壬������Ũ����Ϊ��������Ũ����Ϊ������ͨ���ױ���������Ӧ�Ʊ���
![]()
![]()
![]() +
+ +
+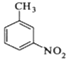
һ���µ��Ʊ��������ױ���ʵ�鷽���ǣ��Է�������Ϊ������������NaHSO4Ϊ����(��ѭ��ʹ��)����CCl4��Һ�У�����������(����ˮ����)��45�淴Ӧ1h ����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ���
(1)����ʵ���й��˵�Ŀ����___________��
(2)��Һ�ڷ�Һ©����ϴ�Ӿ��ú��л��㴦��________��(����������'����)��
(3)5% NaHCO3��Һϴ�ӵ�Ŀ����__________
(4)���и����˴������༰�����Լױ�������ӦӰ���ʵ������
���� | n(����)/n(�ױ�) | ���������и����칹����������(%) | �ܲ���(%) | ||
�������ױ� | �������ױ� | �������ױ� | |||
ŨH2SO4 | 1.0 | 35.6 | 60.2 | 4.2 | 98.0 |
1.2 | 36.5 | 59.5 | 4.0 | 99.8 | |
NaHSO4 | 0.15 | 44.6 | 55.1 | 0.3 | 98.9 |
0.25 | 46.3 | 52.8 | 0.9 | 99.9 | |
0.32 | 47.9 | 51.8 | 0.3 | 99.9 | |
0.36 | 45.2 | 54.2 | 0.6 | 99.9 | |
��NaHSO4���Ʊ��������ױ�ʱ��������ױ���������ʵ���֮��Ϊ_______________��
���ɼױ������õ��ĸ��ֲ���ĺ�����֪���ױ�������Ӧ���ص���_________________��
����Ũ������ױ�������ȣ�NaHSO4���ױ��������ŵ���_____________��