��Ŀ����
(14 ��) һ����̼���㷺Ӧ����ұ��ҵ�͵��ӹ�ҵ��
�Ÿ�¯��������Ϊ�ձ��������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
4CO(g)��Fe3O4(s)��4CO2(g)��3Fe(s) ��H="a" kJ��mol��1
CO(g)��3Fe2O3(s)��CO2(g)��2Fe3O4(s) ��H="b" kJ��mol��1
��Ӧ3CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s)�ġ�H= kJ��mol��1(�ú�a��b �Ĵ���ʽ��ʾ)��
�Ƶ��ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����⡢�ֽ�������Ӧ�õ���
��һ����2CH3OH(g) HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0
�ڶ�����HCOOCH3(g) CH3OH(g) +CO(g) ��H>0
CH3OH(g) +CO(g) ��H>0
�ٵ�һ����Ӧ�Ļ�����������ͼ��ʾ��
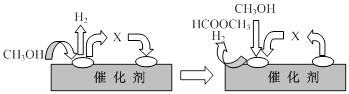
ͼ���м����X�Ľṹ��ʽΪ ��
���ڹ�ҵ�����У�Ϊ���CO�IJ��ʣ��ɲ�ȡ�ĺ�����ʩ�� ��
��Ϊ��������о�����CO��ԭ��������ʯ����Ӧ��������ʵ�X������������ͼ��ͼ��ʾ��X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ������Ӧ�������е�һ�ֲ����������ᷴӦ���������Σ��÷�Ӧ�����ӷ���ʽΪ ��

��ij������Ʒ����Ni2O340%������ΪSiO2��ͨ����ԭ���ᴿ������������ʣ�������CO��33.2 g��Ʒ�ڼ��������»�ԭΪ������Ȼ���ڳ�����ʹ�����е�Ni��CO��ϳ�Ni(CO)4���е�43 �棩������180 ��ʱʹNi(CO)4���·ֽ���������ʡ�
��������������CO�����ʵ���֮��Ϊ ��
��Ϊ��ȫ�������ҵ��������Կ����е�CO���м�⡣
�ٷۺ�ɫ��PdCl2��Һ���Լ��������������CO���������к�CO������Һ�л������ɫ��Pd������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ ��
��ʹ�õ绯ѧһ����̼���崫����������������CO��������ṹ��ͼ��ʾ�����ִ���������ԭ���ԭ������õ�صĸ�����ӦʽΪ ��

�Ÿ�¯��������Ϊ�ձ��������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
4CO(g)��Fe3O4(s)��4CO2(g)��3Fe(s) ��H="a" kJ��mol��1
CO(g)��3Fe2O3(s)��CO2(g)��2Fe3O4(s) ��H="b" kJ��mol��1
��Ӧ3CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s)�ġ�H= kJ��mol��1(�ú�a��b �Ĵ���ʽ��ʾ)��
�Ƶ��ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����⡢�ֽ�������Ӧ�õ���
��һ����2CH3OH(g)
 HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0�ڶ�����HCOOCH3(g)
 CH3OH(g) +CO(g) ��H>0
CH3OH(g) +CO(g) ��H>0�ٵ�һ����Ӧ�Ļ�����������ͼ��ʾ��

ͼ���м����X�Ľṹ��ʽΪ ��
���ڹ�ҵ�����У�Ϊ���CO�IJ��ʣ��ɲ�ȡ�ĺ�����ʩ�� ��
��Ϊ��������о�����CO��ԭ��������ʯ����Ӧ��������ʵ�X������������ͼ��ͼ��ʾ��X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ������Ӧ�������е�һ�ֲ����������ᷴӦ���������Σ��÷�Ӧ�����ӷ���ʽΪ ��

��ij������Ʒ����Ni2O340%������ΪSiO2��ͨ����ԭ���ᴿ������������ʣ�������CO��33.2 g��Ʒ�ڼ��������»�ԭΪ������Ȼ���ڳ�����ʹ�����е�Ni��CO��ϳ�Ni(CO)4���е�43 �棩������180 ��ʱʹNi(CO)4���·ֽ���������ʡ�
��������������CO�����ʵ���֮��Ϊ ��
��Ϊ��ȫ�������ҵ��������Կ����е�CO���м�⡣
�ٷۺ�ɫ��PdCl2��Һ���Լ��������������CO���������к�CO������Һ�л������ɫ��Pd������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ ��
��ʹ�õ绯ѧһ����̼���崫����������������CO��������ṹ��ͼ��ʾ�����ִ���������ԭ���ԭ������õ�صĸ�����ӦʽΪ ��

��(2a+b)/3 �Ƣ�HCHO �������¶ȣ�����ѹǿ
��FeAl2O4+8H+��Fe2++2Al3++4H2O ��3:8
�ɢ�0.1mol����0.1NA�� ��CO+H2O��2e����CO2+2H+��ÿ��2�֣���14�֣�
��FeAl2O4+8H+��Fe2++2Al3++4H2O ��3:8
�ɢ�0.1mol����0.1NA�� ��CO+H2O��2e����CO2+2H+��ÿ��2�֣���14�֣�
����������Ž����١�
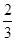 +�ڡ�
+�ڡ�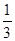 ���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1��
���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1���Ƣ�CH3OH���������HCHO��
�ڸ��ݵ�һ���͵ڶ����ķ�Ӧ�ص㣬��֪�������¶ȣ�����ѹǿ�������CO�IJ��ʣ�
�Ǹ��ݡ���Ӧ�������е�һ�ֲ����������ᷴӦ���������Ρ�˵���÷�Ӧ�ķ�Ӧ����FeAl2O4�����ᣬ��������FeCl2��AlCl3��H2O���ݴ˱��д�����ӷ���ʽ��
����ȷ��������������Ӧ��3CO+Ni2O3��3CO2+2Ni��Ni+4CO��Ni(CO)4����������������CO�����ʵ���֮��Ϊ3��8��
�ɢٸ��ݡ�PdCl2��Pd��2e-������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ
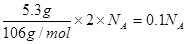 ��
����CO�ڸ����Ϸ���������Ӧ���ݵ�ʧ�����غ���CO��2e�D�D�DCO2���پݵ���غ���CO��2e�D�DCO2+2H+������ԭ���غ��CO+H2O��2e����CO2+2H+��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 2H2����O2��
2H2����O2�� CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 ��ԭΪN2��һ��ʱ�����Һ�ļ���
��ԭΪN2��һ��ʱ�����Һ�ļ���

